Diabetes Metab J.
2011 Jun;35(3):226-235. 10.4093/dmj.2011.35.3.226.
Comparison of EGF with VEGF Non-Viral Gene Therapy for Cutaneous Wound Healing of Streptozotocin Diabetic Mice
- Affiliations
-
- 1Molecular Therapy Laboratory, Paik Memorial Institute for Clinical Research, Busan, Korea. pjhdoc@chol.com
- 2Department of Internal Medicine, Inje University College of Medicine, Busan, Korea.
- 3Department of Pathology, Inje University College of Medicine, Busan, Korea.
- 4Department of Internal Medicine, Maryknoll Medical Center, Busan, Korea.
- KMID: 2175423
- DOI: http://doi.org/10.4093/dmj.2011.35.3.226
Abstract
- BACKGROUND
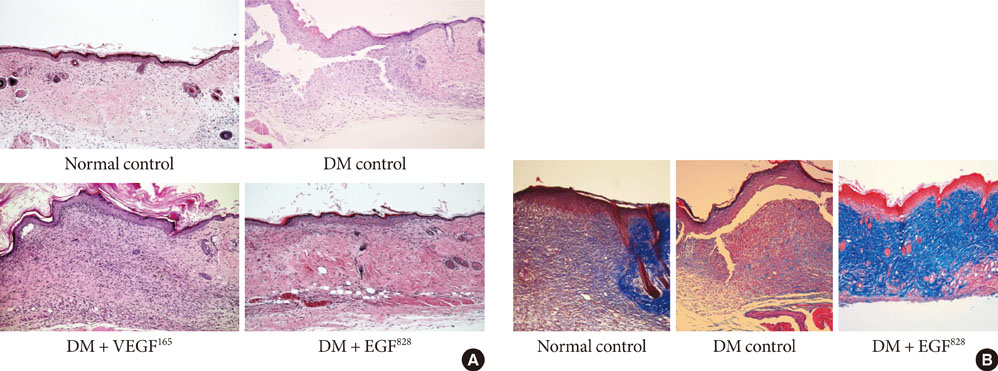
To accelerate the healing of diabetic wounds, various kinds of growth factors have been employed. It is the short half-life of administered growth factors in hostile wound beds that have limited wide-spread clinical usage. To overcome this limitation, growth factor gene therapy could be an attractive alternative rather than direct application of factors onto the wound beds. We administered two growth factor DNAs, epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) into a cutaneous wound on diabetic mice. We compared the different characteristics of the healing wounds.
METHODS
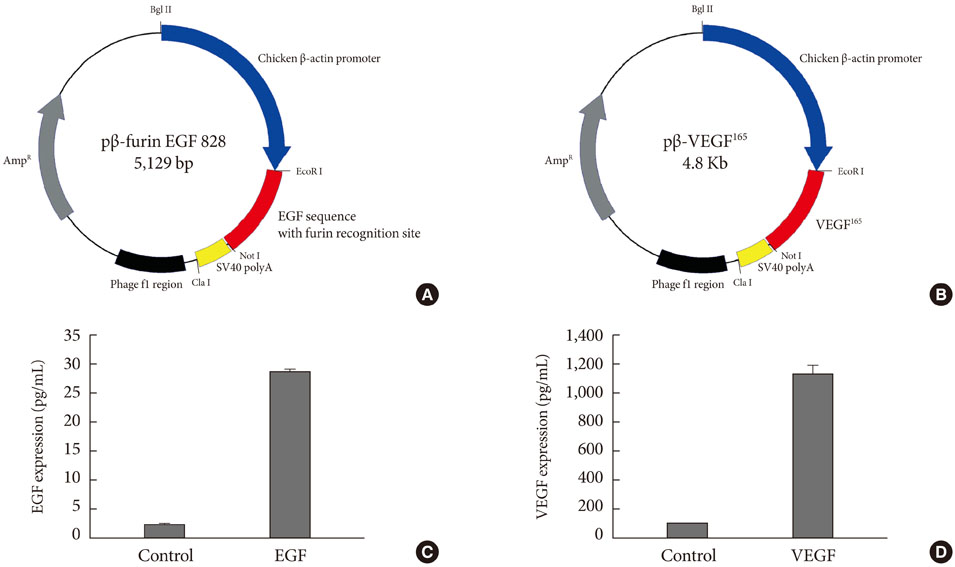
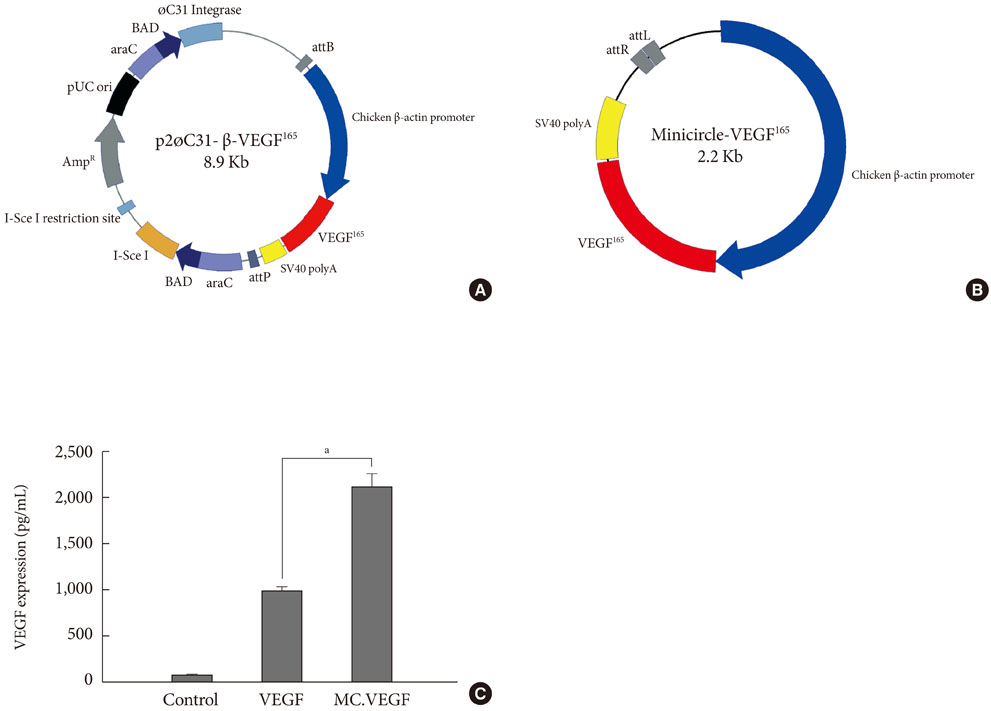
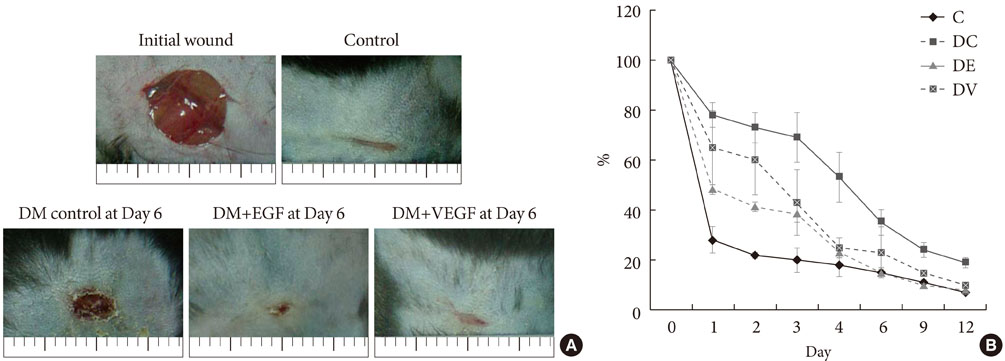
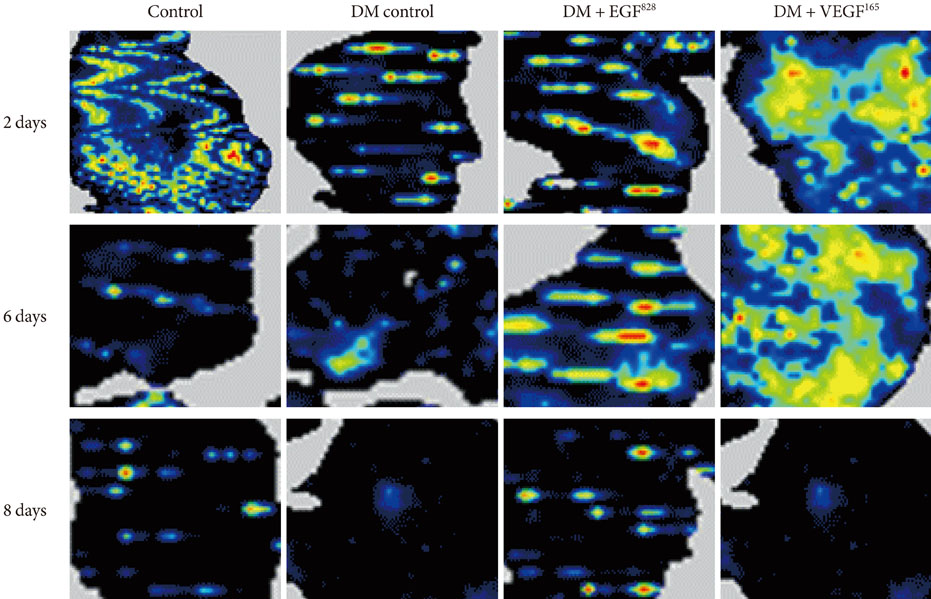
Streptozotocin was injected intraperitoneally to induce diabetes into C57BL/6J mice. The ultrasound micro-bubble destruction method with SonoVue as a bubbling agent was used for non-viral gene delivery of EGF828 and VEGF165 DNAs. Each gene was modified for increasing efficacy as FRM-EGF828 or minicircle VEGF165. The degree of neoangiogenesis was assessed using qualitative laser Doppler flowmetry. We compared wound size and histological findings of the skin wounds in each group.
RESULTS
In both groups, accelerated wound closure was observed in the mice receiving gene therapy compared with non treated diabetic control mice. Blood flow detected by laser doppler flowmetry was better in the VEGF group than in the EGF group. Wound healing rates and histological findings were more accelerated in the EGF gene therapy group than the VEGF group, but were not statistically significant.
CONCLUSION
Both non-viral EGF and VEGF gene therapy administrations could improve the speed and quality of skin wound healing. However, the detailed histological characteristics of the healing wounds were different.
Keyword
MeSH Terms
-
Animals
DNA
Epidermal Growth Factor
Genetic Therapy
Half-Life
Intercellular Signaling Peptides and Proteins
Laser-Doppler Flowmetry
Mice
Phospholipids
Skin
Streptozocin
Sulfur Hexafluoride
Vascular Endothelial Growth Factor A
Wound Healing
DNA
Epidermal Growth Factor
Intercellular Signaling Peptides and Proteins
Phospholipids
Streptozocin
Sulfur Hexafluoride
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg. 2003. 90:133–146.2. Petri JB, Konig S, Haupt B, Haustein UF, Herrmann K. Molecular analysis of different phases in human wound healing. Exp Dermatol. 1997. 6:133–139.3. Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol. 1998. 111:850–857.4. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008. 16:585–601.5. Hantash BM, Zhao L, Knowles JA, Lorenz HP. Adult and fetal wound healing. Front Biosci. 2008. 13:51–61.6. Cohen S, Elliott GA. The stimulation of epidermal keratinization by a protein isolated from the submaxillary gland of the mouse. J Invest Dermatol. 1963. 40:1–5.7. Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem. 1990. 265:7709–7712.8. Shiraha H, Glading A, Gupta K, Wells A. IP-10 inhibits epidermal growth factor-induced motility by decreasing epidermal growth factor receptor-mediated calpain activity. J Cell Biol. 1999. 146:243–254.9. Schultz G, Rotatori DS, Clark W. EGF and TGF-alpha in wound healing and repair. J Cell Biochem. 1991. 45:346–352.10. Brown GL, Curtsinger L 3rd, Brightwell JR, Ackerman DM, Tobin GR, Polk HC Jr, George-Nascimento C, Valenzuela P, Schultz GS. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med. 1986. 163:1319–1324.11. Brown GL, Curtsinger LJ, White M, Mitchell RO, Pietsch J, Nordquist R, von Fraunhofer A, Schultz GS. Acceleration of tensile strength of incisions treated with EGF and TGF-beta. Ann Surg. 1988. 208:788–794.12. Namiki A, Brogi E, Kearney M, Kim EA, Wu T, Couffinhal T, Varticovski L, Isner JM. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J Biol Chem. 1995. 270:31189–31195.13. Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998. 152:1445–1452.14. Banks RE, Forbes MA, Kinsey SE, Stanley A, Ingham E, Walters C, Selby PJ. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer. 1998. 77:956–964.15. Gaudry M, Bregerie O, Andrieu V, El Benna J, Pocidalo MA, Hakim J. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood. 1997. 90:4153–4161.16. Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell. 1992. 3:211–220.17. Jazwa A, Loboda A, Golda S, Cisowski J, Szelag M, Zagorska A, Sroczynska P, Drukala J, Jozkowicz A, Dulak J. Effect of heme and heme oxygenase-1 on vascular endothelial growth factor synthesis and angiogenic potency of human keratinocytes. Free Radic Biol Med. 2006. 40:1250–1263.18. Yebra M, Parry GC, Stromblad S, Mackman N, Rosenberg S, Mueller BM, Cheresh DA. Requirement of receptor-bound urokinase-type plasminogen activator for integrin alphavbeta5-directed cell migration. J Biol Chem. 1996. 271:29393–29399.19. Suzuma K, Takagi H, Otani A, Honda Y. Hypoxia and vascular endothelial growth factor stimulate angiogenic integrin expression in bovine retinal microvascular endothelial cells. Invest Ophthalmol Vis Sci. 1998. 39:1028–1035.20. Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol. 1996. 149:293–305.21. Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, Ziche M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol. 1996. 270(1 Pt 2):H411–H415.22. Pepper MS, Ferrara N, Orci L, Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992. 189:824–831.23. Goto F, Goto K, Weindel K, Folkman J. Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels. Lab Invest. 1993. 69:508–517.24. Watanabe Y, Lee SW, Detmar M, Ajioka I, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) delays and induces escape from senescence in human dermal microvascular endothelial cells. Oncogene. 1997. 14:2025–2032.25. Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998. 273:30336–30343.26. Yoon CS, Jung HS, Kwon MJ, Lee SH, Kim CW, Kim MK, Lee M, Park JH. Sonoporation of the minicircle-VEGF(165) for wound healing of diabetic mice. Pharm Res. 2009. 26:794–801.27. Ramabadran K, Bansinath M, Turndorf H, Puig MM. The hyperalgesic effect of naloxone is attenuated in streptozotocin-diabetic mice. Psychopharmacology (Berl). 1989. 97:169–174.28. Anjaneyulu M, Ramarao P. Studies on gastrointestinal tract functional changes in diabetic animals. Methods Find Exp Clin Pharmacol. 2002. 24:71–75.29. Martin P. Wound healing: aiming for perfect skin regeneration. Science. 1997. 276:75–81.30. Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med. 2004. 351:48–55.31. Setacci C, de Donato G, Setacci F, Chisci E. Diabetic patients: epidemiology and global impact. J Cardiovasc Surg (Torino). 2009. 50:263–273.32. Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003. 60:107–114.33. Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999. 77:527–543.34. Rosengart TK, Lee LY, Patel SR, Kligfield PD, Okin PM, Hackett NR, Isom OW, Crystal RG. Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA. Ann Surg. 1999. 230:466–470.35. Brem H, Kodra A, Golinko MS, Entero H, Stojadinovic O, Wang VM, Sheahan CM, Weinberg AD, Woo SL, Ehrlich HP, Tomic-Canic M. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J Invest Dermatol. 2009. 129:2275–2287.36. Acosta JB, Savigne W, Valdez C, Franco N, Alba JS, del Rio A, Lopez-Saura P, Guillen G, Lopez E, Herrera L, Fernandez-Montequin J. Epidermal growth factor intralesional infiltrations can prevent amputation in patients with advanced diabetic foot wounds. Int Wound J. 2006. 3:232–239.37. Jazwa A, Kucharzewska P, Leja J, Zagorska A, Sierpniowska A, Stepniewski J, Kozakowska M, Taha H, Ochiya T, Derlacz R, Vahakangas E, Yla-Herttuala S, Jozkowicz A, Dulak J. Combined vascular endothelial growth factor-A and fibroblast growth factor 4 gene transfer improves wound healing in diabetic mice. Genet Vaccines Ther. 2010. 8:6.38. Andreadis ST, Geer DJ. Biomimetic approaches to protein and gene delivery for tissue regeneration. Trends Biotechnol. 2006. 24:331–337.39. Vasita R, Katti DS. Growth factor-delivery systems for tissue engineering: a materials perspective. Expert Rev Med Devices. 2006. 3:29–47.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cloning of Novel Epidermal Growth Factor (EGF) Plasmid for Gene Therapy on Diabetic Foot Ulcer
- Comparison of Minicircle with Conventional Plasmid for the Non-viral Vascular Endothelial Growth Factor (VEGF) Gene Therapy
- Polysaccharides isolated from Phellinus gilvus enhances dermal wound healing in streptozotocin-induced diabetic rats
- Effects of Human Adipose-derived Stem Cells on Cutaneous Wound Healing in Nude Mice
- The Effect of Epidermal Growth Factor on Cornea Epithelial Wound Healing in Excimer Laser Keratectomized Rabbits