J Korean Acad Conserv Dent.
2006 May;31(3):203-214. 10.5395/JKACD.2006.31.3.203.
Anti-inflammatory effects of PPARgamma on human dental pulp cells
- Affiliations
-
- 1Department of Conservative Dentistry, College of Dentistry, Chonbuk National University, Korea. endo95@naver.com
- KMID: 2175820
- DOI: http://doi.org/10.5395/JKACD.2006.31.3.203
Abstract
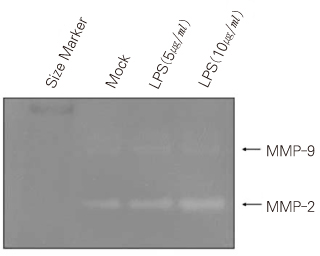
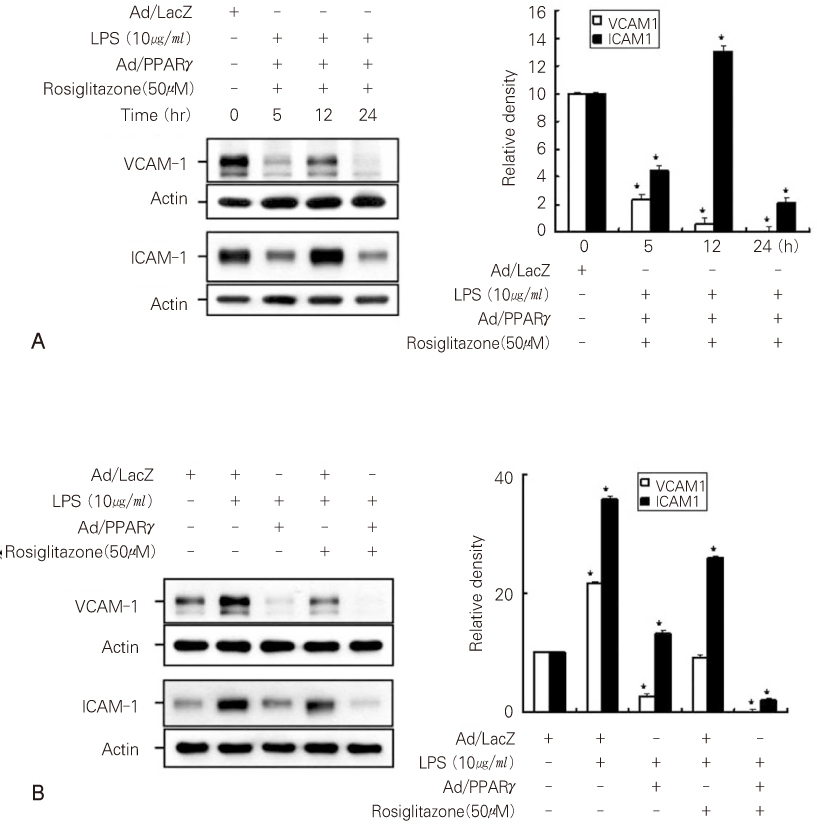
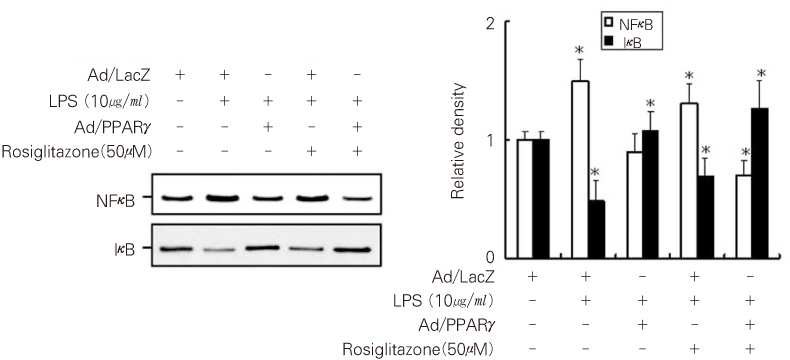
- Dental pulp is a loose, mesenchymal tissue almost entirely enclosed in the dentin. It consists of cells, ground substance, and neural and vascular supplies. Damage to the dental pulp by mechanical, chemical, thermal, and microbial irritants can provoke various types of inflammatory response. Pulpal inflammation leads to the tissue degradation, which is mediated in part by Matrix metalloproteinase leads to accelerate extracellular matrix degradation with pathological pathway. We have now investigated the induction of MMPs and inflammatory cytokines by Lipopolysaccharide (LPS) control of inflammatory mediators by peroxisome proliferator-activated receptors (PPARs). Human dental pulp cells exposed to various concentrations of LPS (1-10 microg/ml) revealed elevated levels of MMP-2 and MMP-9 at 24 hrs of culture. LPS also stimulated the production of ICAM-1, VCAM-1, IL-1beta, and TNF-alpha. Adenovirus PPARgamma (Ad/PPARgamma) and PPARgamma agonist rosiglitazone reduced the synthesis of MMPs, adhesion molecules and pro-inflammatory cytokines. The inhibitory effect of Ad/PPARgamma was higher than that of PPARgamma agonist. These result offer new insights in regard to the anti-inflammatory potential of PPARgamma in human dental pulp cell.
Keyword
MeSH Terms
-
Adenoviridae
Cytokines
Dental Pulp*
Dentin
Equipment and Supplies
Extracellular Matrix
Humans*
Inflammation
Intercellular Adhesion Molecule-1
Irritants
Matrix Metalloproteinases
Peroxisome Proliferator-Activated Receptors
PPAR gamma*
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Cytokines
Intercellular Adhesion Molecule-1
Irritants
Matrix Metalloproteinases
PPAR gamma
Peroxisome Proliferator-Activated Receptors
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Figure
Reference
-
1. Brandenburg K, Andra J, Muller M, Koch MH, Garidel P. Physicochemical properties of bacterial glycopolymers in relation to bioactivity. Carbohydr Res. 2003. 338(23):2477–2489.
Article2. Birkedal-Hansen H. Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res. 1993. 28:500–510.
Article3. Simonin MA, Bordji K. PPAR-γ ligands modulate effects of LPS in stimulated rat synovial fibroblasts. Am J Physiol Cell Physiol. 2002. 282:C125–C133.4. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003. 92(8):827–839.5. Naito Y, Yoshikawa T. Role of matrix metalloproteinases in inflammatory bowel disease. Mol Aspects Med. 2005. 26:379–390.
Article6. Gusman H, Santana RB, Zehnder M. Matrix metalloproteinase levels and gelatinolytic activity in clinically healthy and inflamed human dental pulps. Eur J Oral Sci. 2002. 110(5):353–357.
Article7. Wang CY, Stashenko P. Kinetics of bone-resorbing activity in developing periapical lesion. J Dent Res. 1991. 70:1362–1366.
Article8. Zhu X, Subbaraman R, Sano H, Jacobs B, Sano A, Boetticher E, Munoz NM, Leff AR. A surrogate method for assessment of beta(2)-integrin-dependent adhesion of human eosinophils to ICAM-1. J Immunol Methods. 2000. 240(1-2):157–164.
Article9. Sawa Y, Yoshida S, Shibata KI, Suzuki M, Mukaida A. Vascular endothelium of human dental pulp expresses diverse adhesion molecules for leukocyte emigration. Tissue Cell. 1998. 30(2):281–291.
Article10. Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta. 1996. 1302:93–109.
Article11. Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibits production of monocyte inflammatory cytokines. Nature. 1998. 391:82–86.
Article12. Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000. 43:527–550.
Article13. Lemberger T, Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: a nuclear receptor signaling pathway in lipid physiology. Annu Rev Cell Dev Biol. 1996. 12:335–363.
Article14. Panagakos FS, O'Boskey JF Jr, Rodriguez E. Regulation of pulp cell matrix metalloproteinase production by cytokines and lipopolysaccharides. J Endod. 1996. 22:358–361.
Article15. Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-gamma is a new therapeutic target in sepsis and inflammation. Shock. 2005. 23(5):393–399.
Article16. Tjäderhane L. The mechanism of pulpal wound healing. Aust Endod J. 2002. 28(2):68–74.
Article17. Lin SK, Wang CC, Huang S, Lee JJ, Chiang CP, Lan WH, Hong CY. Induction of dental pulp fibroblast matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 gene expression by interleukin-1alpha and tumor necrosis factor-alpha through a prostaglandin-dependent pathway. J Endod. 2001. 27:185–189.
Article18. Coil J, Tam E, Waterfield JD. Proinflammatory cytokine profiles in pulp fibroblasts stimulated with lipopolysaccharide and methyl mercaptan. J Endod. 2004. 30(2):88–91.
Article19. Min JK, Kim YM, Kim SW, Kwon MC, Kong YY, Hwang IK, Won MH, Rho J, Kwon YG. TNF-related activation-induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-kappaB activation in endothelial cells. J Immunol. 2005. 175(1):531–540.
Article20. Sasaki M, Jorden P, Welbourne T, Minagar A, Joh T, Itoh M, Elrod JW, Alexander JS. Troglitazone, a PPAR-gamma activator prevents endothelial cell adhesion molecule expression and lymphocyte adhesion mediated by TNF-alpha. BMC Physiol. 2005. 5(1):3–14.21. Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000. 101(3):235–238.
Article22. Ward JE, Fernades DJ, Taylor CC, Bonacci JV, Quan L, Stewart AG. The PPARgamma ligand, rosiglitazone, reduces airways hyperresponsiveness in a murine model of allergen-induced inflammation. Pulm Pharmacol Ther. 2006. 19(1):39–46.
Article23. Madianos PN, Bobetsis YA, Kinane DF. Generation of inflammatory stimuli: how bacteria set up inflammatory responses in the gingiva. J Clin Periodontol. 2005. 32:57–71.
Article24. Yang WK, Lee WC, Kim MR, Son HH. MMP and TIMP production in periodontal ligament fibroblasts stimulated by prevotella nigrescens lipopolysaccharide. J Korean Acad Conserv Dent. 2005. 30(5):372–384.
Article25. Park SK, Shon WJ, Lim SS. Effect of Sonicated extracts of Enterococcus feacalis on the production of Matrix Metalloproteinase-8 by human polymorphonuclear neutrophils. J Korean Acad Conserv Dent. 2005. 30(2):138–144.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dlx3 and Dlx5 Inhibit Adipogenic Differentiation of Human Dental Pulp Stem Cells
- Stem cell-derived exosomes for dentin-pulp complex regeneration: a mini-review
- Dental Pulp Stem Cells and Current in vivo Approaches to Study Dental Pulp Stem Cells in Pulp Injury and Regeneration
- Effect of hypoxia on angiogenesis-related proteins in human dental pulp cells
- Characterization of Human Dental Pulp Cells from Supernumerary Teeth by Using Flow Cytometry Analysis