J Breast Cancer.
2007 Mar;10(1):43-50. 10.4048/jbc.2007.10.1.43.
Role of Zoledronic Acid on Bone Loss by Letrozole
- Affiliations
-
- 1Breast Center, KangNam St. Mary's Hospital, Department of Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea. ssjung@catholic.ac.kr
- 2Department of Pathology, Ilsan Paik Hospital, College of Medicine, Inje University, Goyang, Korea.
- KMID: 2175000
- DOI: http://doi.org/10.4048/jbc.2007.10.1.43
Abstract
-
PURPOSE: The aromatase inhibitors cause bone loss by estrogen depletion. Zoledronic acid (ZA) can prevent bone mineral density (BMD) loss associated with the use of aromatase inhibitors. Accordingly interest has arisen in measuring surrogate markers of bone resorption to monitor the response of treatment of BMD loss in place of a radiologic assessment. This study was designed to determine whether ZA would prevent bone loss that is known to occur with letrozole and identified surrogate markers of bone resorption in an animal model.
METHODS
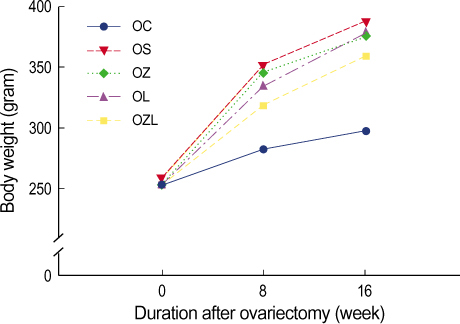
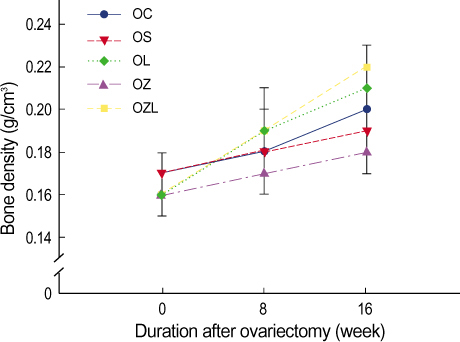
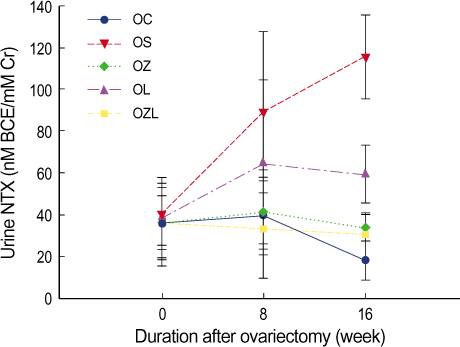
In ovariectomized or sham-operated rat, we administrated ZA and letrozole to 5 different groups including: a sham operation control group (OC), a group in which an ovariectomy was performed followed by saline administration (OS), an ovariectomy with ZA treatment group (OZ), an ovariectomy with letrozole treatment group (OL) and an ovariectomy with ZA and letrozole combined treatment group (OZL). The levels of serum osteocalcin, serum bone alkaline phosphatase (BALP), serum calcium and urine N-telopeptide (NTX) and BMD were estimated and compared at the same periods for each group. The distinct microscopic findings of proximal tibia at week sixteen were also compared.
RESULTS
Significantly reduced levels of urine NTX and significantly increased BMD were measured in the OZ group. In the OL group no difference was seen in in BMD in comparison to the OS group. However, a significant increase in BMD was measured in the OZL group. Urine NTX levels were measured and found to be lower in the OL group and significantly lower in the OZL group. Serum osteocalcin levels were similar to each other for each group. Levels of serum calcium and BALP were significantly lower in the OZL group than in the OS group.
CONCLUSION
The combination treatment with ZA and letrozole is effective in the inhibition of bone resorption and in the preservation of BMD. Measurement of serum osteocalcin, urine NTX, and BMD, levels are recommended as surrogate markers for determining the response for the treatment of bone loss.
MeSH Terms
Figure
Reference
-
1. Baum M, Buzdar A, Cuzick J, Forbes J, Houghton J, Howell A, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003. 98:1802–1810.
Article2. Mackey JR, Joy AA. Skeletal health in postmenopausal survivors of early breast cancer. Int J Cancer. 2005. 114:1010–1015.
Article3. Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005. 23:619–629.
Article4. Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004. 350:1081–1092.
Article5. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003. 349:1793–1802.
Article6. Harvey HA. Optimizing bisphosphonate therapy in patients with breast cancer on endocrine therapy. Semin Oncol. 2004. 31:23–30.
Article7. Delea T, Langer C, McKiernan J, Liss M, Edelsberg J, Brandman J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology. 2004. 67:390–396.
Article8. Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003. 21:4042–4057.
Article9. Lipton A, Theriault RL, Hortobagyi GN, Simeone J, Knight RD, Mellars K, et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000. 88:1082–1090.
Article10. Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995. 10:1478–1487.
Article11. Lowik CW, van der Pluijm G, van der Wee-Pals LJ, van Treslong-De Groot HB, Bijvoet OL. Migration and phenotypic transformation of osteoclast precursors into mature osteoclasts: the effect of a bisphosphonate. J Bone Miner Res. 1988. 3:185–192.
Article12. Suzuki K, Takeyama S, Sakai Y, Yamada S, Shinoda H. Current topics in pharmacological research on bone metabolism: inhibitory effects of bisphosphonates on the differentiation and activity of osteoclasts. J Pharmacol Sci. 2006. 100:189–194.
Article13. Green JR. Antitumor effects of bisphosphonates. Cancer. 2003. 97:840–847.
Article14. Green JR, Clezardin P. Mechanisms of bisphosphonate effects on osteoclasts, tumor cell growth, and metastasis. Am J Clin Oncol. 2002. 25:S3–S9.
Article15. Coleman RE. The clinical use of bone resorption markers in patients with malignant bone disease. Cancer. 2002. 94:2521–2533.
Article16. Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005. 23:4925–4935.
Article17. Christenson RH. Biochemical markers of bone metabolism: an overview. Clin Biochem. 1997. 30:573–593.
Article18. Bonde M, Qvist P, Fledelius C, Riis BJ, Christiansen C. Immunoassay for quantifying type I collagen degradation products in urine evaluated. Clinical Chemistry. 1994. 40:2022–2025.
Article19. Behr W, Barnert J. Quantification of bone alkaline phosphatase in serum by precipitation with wheat-germ lectin: a simplified method and its clinical plausibility. Clinical Chemistry. 1986. 32:1960–1966.
Article20. Rosalki SB, Foo AY. Two new methods for separating and quantifying bone and liver alkaline phosphatase isoenzymes in plasma. Clinical Chemistry. 1984. 30:1182–1186.
Article21. Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002. 346:653–661.
Article22. Brufsky A, Harker G, Beck T, Carroll R, Tan-Chiu E, Seidler C, et al. Zoledronic acid (ZA) for prevention of cancer treatment-induced bone loss (CTIBL) in postmenopausal women (PMW) with early breast cancer (BCa) receiving adjuvant Letrozole (Let): Preliminary results of the Z-FAST trial. 2004. Presented at the 2004 San Antonio Breast Cancer Symposium. [Abstract 1114].23. Brufsky A, Harker W, Beck J, Carroll R, Tan-Chiu E, Seidler C, et al. Zoledronic acid (ZA) effectively inhibits cancer treatment-induced bone loss (CTIBL) in postmenopausal women (PMW) with early breast cancer (BCa) receiving adjuvant Letrozole (Let): 12 mos BMD results of the Z-FAST trial. Proc Am Soc Clin Oncol. 2005. 23:12.
Article24. Davidson N, Neven P, Llombart A, Bundred N, Monnier A, DeBoer R, et al. Zoledronic acid in the prevention of cancer treatment-induced bone loss in postmenopausal women receiving letrozole as adjuvant therapy for early breast cancer. Breast. 2005. 14:44.25. Duda RJ Jr, O'Brien JF, Katzmann JA, Peterson JM, Mann KG, Riggs BL. Concurrent assays of circulating bone Gla-protein and bone alkaline phosphatase: effects of sex, age, and metabolic bone disease. J Clin Endocrinol Metab. 1988. 66:951–957.
Article26. Coleman RE, Mashiter G, Fogelman I, Whitaker KD, Caleffi M, Moss DW, et al. Osteocalcin: a potential marker of metastatic bone disease and response to treatment. Eur J Cancer Clin Oncol. 1988. 24:1211–1217.
Article27. Heaney RP. How does bone support calcium homeostasis? Bone. 2003. 33:264–268.
Article28. Ueda M, Inaba M, Okuno S, Maeno Y, Ishimura E, Yamakawa T, et al. Serum BAP as the clinically useful marker for predicting BMD reduction in diabetic hemodialysis patients with low PTH. Life Sci. 2005. 77:1130–1139.
Article29. Dresner-Pollak R, Mayer M, Hochner-Celiniker D. The decrease in serum bone-specific alkaline phosphatase predicts bone mineral density response to hormone replacement therapy in early postmenopausal women. Calcif Tissue Int. 2000. 66:104–107.
Article30. Woitge HW, Pecherstorfer M, Li Y, Keck AV, Horn E, Ziegler R, et al. Novel serum markers of bone resorption: clinical assessment and comparison with established urinary indices. J Bone Miner Res. 1999. 14:792–801.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preventive Effects of Zoledronic Acid on Bone Metastasis in Mice Injected with Human Breast Cancer Cells
- Atypical Subtrochanteric Femoral Fracture in a Patient with Non-metatatic Breast Cancer on Zoledronic Acid Therapy: A Case Report
- Insufficient Mitigation of Bone Loss by Zoledronic Acid after Treatment with Denosumab
- Intravenous Zoledronic Acid in Elderly Patients with Benign Paroxysmal Positional Vertigo and Osteoporosis
- An Atypical Subtrochanteric Femoral Fracture in a Patient with Multiple Myeloma Received Zoledronic Acid: A Case Report