J Korean Med Sci.
2011 Dec;26(12):1569-1575. 10.3346/jkms.2011.26.12.1569.
Preventive Effects of Zoledronic Acid on Bone Metastasis in Mice Injected with Human Breast Cancer Cells
- Affiliations
-
- 1Department of Surgery, College of Medicine, Yonsei University, Seoul, Korea. hdlee@yuhs.ac
- 2Department of Veterinary Medicine, Konkuk University, Seoul, Korea.
- KMID: 1786012
- DOI: http://doi.org/10.3346/jkms.2011.26.12.1569
Abstract
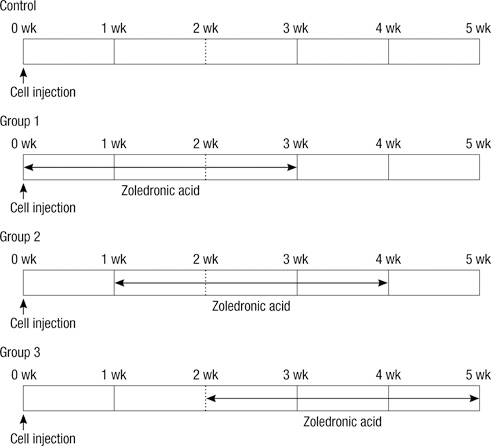
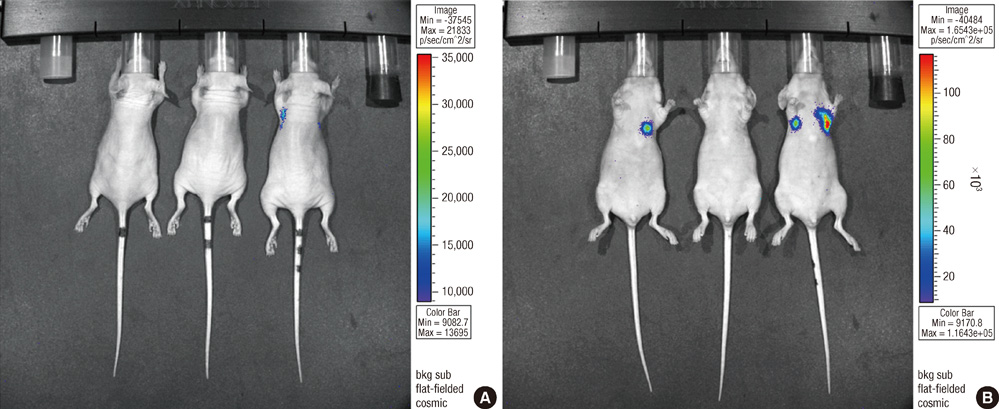
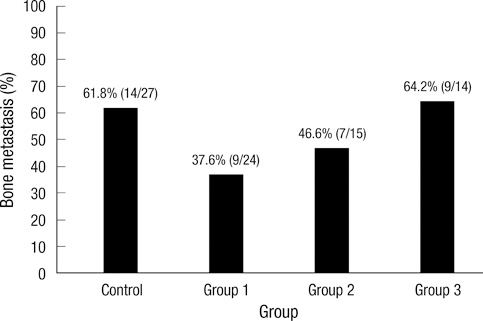
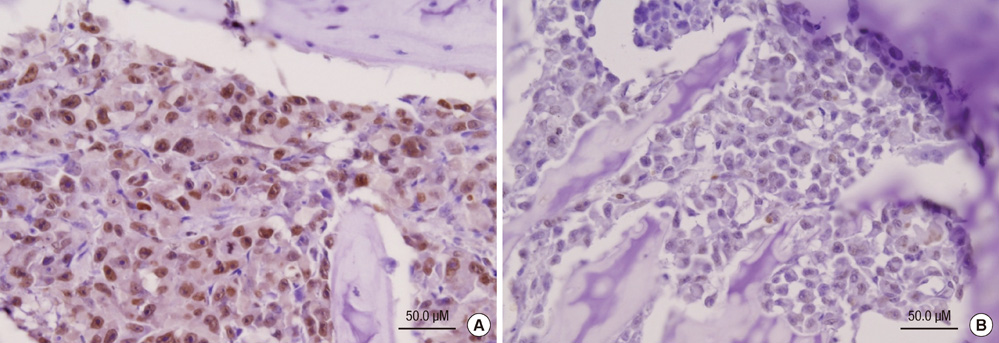
- Bisphosphonates are used routinely to reduce bone-related events in breast cancer patients with bone metastasis. We evaluated the effects of zoledronic acid, a third generation, nitrogen-containing bisphosphonate, to prevent bone metastasis in breast cancer. Zoledronic acid or vehicle alone was administered to nude mice either simultaneously or after intracardiac injection of human breast cancer MDA-MB-231 cells. Nude mice treated with zoledronic acid at early time points showed a lower incidence of bone metastases than did vehicle-treated nude mice, but these differences were not statistically significant. Only 37.5% of mice treated with zoledronic acid at the time of tumor cell inoculation developed bone metastases compared to over 51.8% of mice receiving vehicle alone (P = 0.304). Cell count of apoptosis confirmed by immunohistochemical staining in metastatic bone tissue significantly increased in the zoledronic acid-treated groups compared to non-treated group (1,018.3 vs 282.0; P = 0.046). However, metastatic tumor cells, which invade soft tissue around the bone, did not show extensive apoptosis; there were no differences between the zoledronic acid-treated and control groups. These results suggest that zoledronic acid increases apoptosis of metastatic breast tumor cells in the bone and could therefore reduce metastatic tumor burden. These results support the use of zoledronic acid to reduce the incidence of bone metastasis in breast cancer.
Keyword
MeSH Terms
-
Animals
Apoptosis/drug effects
Bone Density Conservation Agents/pharmacology
Bone Neoplasms/prevention & control/*secondary
Bone and Bones/drug effects/pathology
Breast Neoplasms/*drug therapy/*pathology
Diphosphonates/*pharmacology
Female
Humans
Imidazoles/*pharmacology
Mice
Mice, Nude
Xenograft Model Antitumor Assays
Figure
Cited by 1 articles
-
Survival Benefit of Zoledronic Acid in Postmenopausal Breast Cancer Patients Receiving Aromatase Inhibitors
Sung Gwe Ahn, Sung Hyun Kim, Hak Min Lee, Seung Ah Lee, Joon Jeong
J Breast Cancer. 2014;17(4):350-355. doi: 10.4048/jbc.2014.17.4.350.
Reference
-
1. Rubens RD. Bone metastases: the clinical problem. Eur J Cancer. 1998. 34:210–213.2. Yoneda T, Sasaki A, Mundy GR. Osteolytic bone metastasis in breast cancer. Breast Cancer Res Treat. 1994. 32:73–84.3. Kanis JA. Bone and cancer: pathophysiology and treatment of metastases. Bone. 1995. 17:101S–105S.4. Body JJ. Bisphosphonates. Eur J Cancer. 1998. 34:263–269.5. Mundy GR. Bisphosphonates as cancer drugs. Hosp Pract (Minneap). 1999. 34:81–84. 88–89. 93–94.6. Boonekamp PM, van der Wee-Pals LJ, van Wijk-van Lennep MM, Thesing CW, Bijvoet OL. Two modes of action of bisphosphonates on osteoclastic resorption of mineralized matrix. Bone Miner. 1986. 1:27–39.7. Fleisch H. Bisphosphonates. Pharmacology and use in the treatment of tumor-induced hypercalcaemic and metastatic bone disease. Drugs. 1991. 42:919–944.8. Carano A, Teitelbaum SL, Konset JD, Schlesinger PH, Blair HC. Bisphosphonates directly inhibit the bone resorption activity of isolated avian osteoclasts in vitro. J Clin Invest. 1990. 85:456–461.9. Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991. 88:2095–2105.10. Frijlink WB, Bijvoet OL, Te Velde J, Heynen G. Treatment of Paget's disease with (3-amino-1-hydroxypropylidene)-1, 1-bisphosphonate (A.P.D.). Lancet. 1979. 1:799–803.11. Ryan PJ, Sherry M, Gibson T, Fogelman I. Treatment of Paget's disease by weekly infusions of 3-aminohydroxy-propylidene-1, 1-bisphosphonate (APD). Br J Rheumatol. 1992. 31:97–101.12. Kanis JA, O'Rourke N, McCloskey E. Consequences of neoplasia induced bone-resorption and the use of clodronate. Int J Oncol. 1994. 5:713–731.13. Rizzoli R, Buchs B, Bonjour JP. Effect of a single infusion of alendronate in malignant hypercalcaemia: dose dependency and comparison with clodronate. Int J Cancer. 1992. 50:706–712.14. Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, de Souza P, Zheng M, Urbanowitz G, Reitsma D, Seaman JJ. The Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial. J Clin Oncol. 2003. 21:3150–3157.15. Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein M, Coleman RE, Reitsma DJ, Seaman JJ, Chen BL, Ambros Y. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III. double-blind, comparative trial. Cancer J. 2001. 7:377–387.16. Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein MA, Coleman RE, Reitsma DJ, Chen BL, Seaman JJ. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma. Cancer. 2003. 98:1735–1744.17. El Abdaimi K, Dion N, Papavasiliou V, Cardinal PE, Binderup L, Goltzman D, Ste-Marie LG, Kremer R. The vitamin D analogue EB 1089 prevents skeletal metastasis and prolongs survival time in nude mice transplanted with human breast cancer cells. Cancer Res. 2000. 60:4412–4418.18. Sasaki A, Boyce BF, Story B, Wright KR, Chapman M, Boyce R, Mundy GR, Yoneda T. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Res. 1995. 55:3551–3557.19. Sasaki A, Kitamura K, Alcalde RE, Tanaka T, Suzuki A, Etoh Y, Matsumura T. Effect of a newly developed bisphosphonate, YH529, on osteolytic bone metastases in nude mice. Int J Cancer. 1998. 77:279–285.20. El-Abdaimi K, Ste-Marie L, Papavasiliou V, Dion N, Cardinal PE, Huang D, Kremer R. Pamidronate prevents the development of skeletal metastasis in nude mice transplanted with human breast cancer cells by reducing tumor burden within bone. Int J Oncol. 2003. 22:883–890.21. Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, Wesolowski G, Russell RG, Rodan GA, Reszka AA. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci U S A. 1999. 96:133–138.22. Luckman SP, Hughes DE, Coxon FP, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-transitional prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998. 13:581–589.23. Shipman CM, Rogers MJ, Apperley JF, Russell RG, Croucher PI. Bisphosphonate induces apoptosis in human myeloma cell lines: a novel antitumor activity. Br J Haematol. 1997. 98:665–672.24. Shipman CM, Croucher PI, Russell RG, Helfrich MH, Rogers MJ. The bisphosphonate incadronate (YM175) causes apoptosis of human myeloma cells in vitro by inhibiting the mevalonate pathway. Cancer Res. 1998. 58:5294–5297.25. Aparicio A, Cardner A, Tu Y, Savage A, Berenson J, Lichtenstein A. In vitro cytoreductive effects on multiple myeloma cells induced by bisphosphonates. Leukemia. 1998. 12:220–229.26. Hiraga T, Williams PJ, Ueda A, Tamura D, Yoneda T. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin Cancer Res. 2004. 10:4559–4567.27. Daubiné F, Le Gall C, Gasser J, Green J, Clezardin P. Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. J Natl Cancer Inst. 2007. 99:322–330.28. Peyruchaud O, Winding B, Pécheur I, Serre C, Delmas P, Clézardin P. Early detection of bone metastases in a murine model using fluorescent human breast cancer cells: application to the use of the bisphosphonate zoledronic acid in the treatment of osteolytic lesions. J Bone Miner Res. 2001. 16:2027–2034.29. Diel IJ, Solomayer EF, Costa SD, Gollan C, Goerner R, Wallwiener D, Kaufmann M, Bastert G. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998. 339:357–363.30. Saarto T, Vehmanen L, Virkkunen P, Blomqvist C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 2004. 43:650–656.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synergistic Cytotoxic Effects of Zole-dronic Acid and Radiation in the MCF-7 Human Breast Cancer Cell Line
- Atypical Subtrochanteric Femoral Fracture in a Patient with Non-metatatic Breast Cancer on Zoledronic Acid Therapy: A Case Report
- α-Tocopheryl Succinate Inhibits Osteolytic Bone Metastasis of Breast Cancer by Suppressing Migration of Cancer Cells and Receptor Activator of Nuclear Factor-κB Ligand Expression of Osteoblasts
- Spontaneous Acetabular Periprosthetic Fracture in a Patient Continuously Having Zoledronic Acid
- Atypical Subtrochanteric Femur Fracture in Patient with Metastatic Breast Cancer Treated with Zoledronic Acid