J Korean Bone Joint Tumor Soc.
2012 Dec;18(2):99-103. 10.5292/jkbjts.2012.18.2.99.
An Atypical Subtrochanteric Femoral Fracture in a Patient with Multiple Myeloma Received Zoledronic Acid: A Case Report
- Affiliations
-
- 1Department of Orthopedic Surgery, Kyungpook National University Hospital, Korea. ihparkmdrip@gmail.com
- 2Department of Orthopedic Surgery, Seoul National University Bundang Hospital, Korea.
- KMID: 1431678
- DOI: http://doi.org/10.5292/jkbjts.2012.18.2.99
Abstract
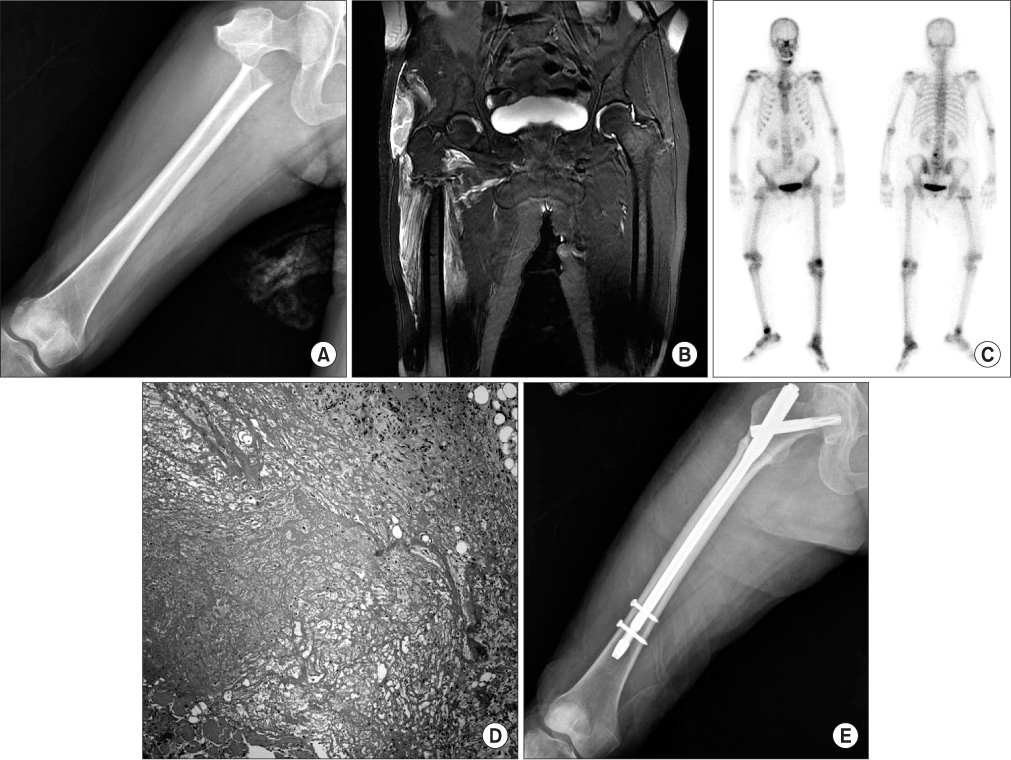
- Little literature exists about the risk of atypical femoral fracture in patients received zoledronic acid for prevention of skeletal metastasis. We report an atypical subtrochanteric femoral fracture in a patient with multiple myeloma received zoledronic acid. The patient was treated by closed reduction and internal fixation with cephalomedullary nailing.
MeSH Terms
Figure
Reference
-
1. Wardley A, Davidson N, Barrett-Lee P, et al. Zoledronic acid significantly improves pain scores and quality of life in breast cancer patients with bone metastases: a randomised, crossover study of community vs hospital bisphosphonate administration. Br J Cancer. 2005. 92:1869–1876.2. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005. 90:1294–1301.3. Body JJ, Bartl R, Burckhardt P, et al. Current use of bisphosphonates in oncology. International Bone and Cancer Study Group. J Clin Oncol. 1998. 16:3890–3899.4. Rosen LS, Gordon D, Tchekmedyian NS, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004. 100:2613–2621.5. Pazianas M, Cooper C, Ebetino FH, Russell RG. Long-term treatment with bisphosphonates and their safety in postmenopausal osteoporosis. Ther Clin Risk Manag. 2010. 6:325–343.6. Kwek EB, Goh SK, Koh JS, Png MA, Howe TS. An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury. 2008. 39:224–231.7. Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000. 15:613–620.8. Kyle RA, Yee GC, Somerfield MR, et al. American Society of Clinical Oncology. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007. 25:2464–2472.9. Van Poznak CH, Temin S, Yee GC, et al. American Society of Clinical Oncology. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol. 2011. 29:1221–1227.10. Puhaindran ME, Farooki A, Steensma MR, Hameed M, Healey JH, Boland PJ. Atypical subtrochanteric femoral fractures in patients with skeletal malignant involvement treated with intravenous bisphosphonates. J Bone Joint Surg Am. 2011. 93:1235–1242.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Atypical Subtrochanteric Femoral Fracture in a Patient with Non-metatatic Breast Cancer on Zoledronic Acid Therapy: A Case Report
- Atypical Subtrochanteric Femur Fracture in Patient with Metastatic Breast Cancer Treated with Zoledronic Acid
- Subtrochanteric Fracture after Cannulatd Screw Fixation of Femoral Neck Fracture in a Child: A Case Report
- Epidemiology and Clinical Features of Atypical Femoral Fractures
- Bilateral Subtrochanteric fracture After Pin Removal in Slipped Capital Femoral Epiphysis: A Case Report