J Gynecol Oncol.
2008 Sep;19(3):173-180. 10.3802/jgo.2008.19.3.173.
Plasma proteomic analysis of patients with squamous cell carcinoma of the uterine cervix
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea. obgynkkt@inje.ac.kr
- 2Paik Institute for Clinical Research, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 3Mitochondrial Signaling Laboratory, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 4Department of Physiology and Biophysics, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- KMID: 2173398
- DOI: http://doi.org/10.3802/jgo.2008.19.3.173
Abstract
OBJECTIVE
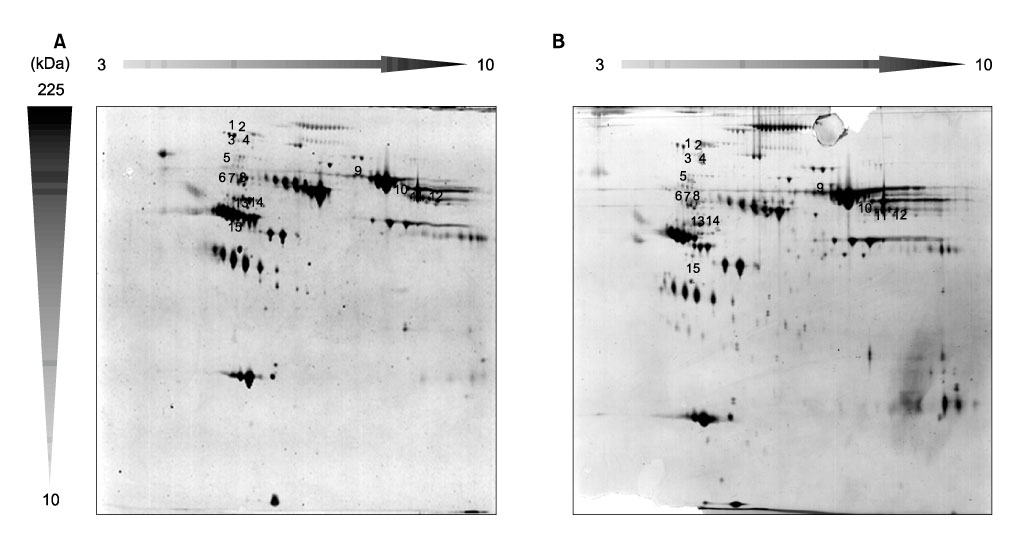
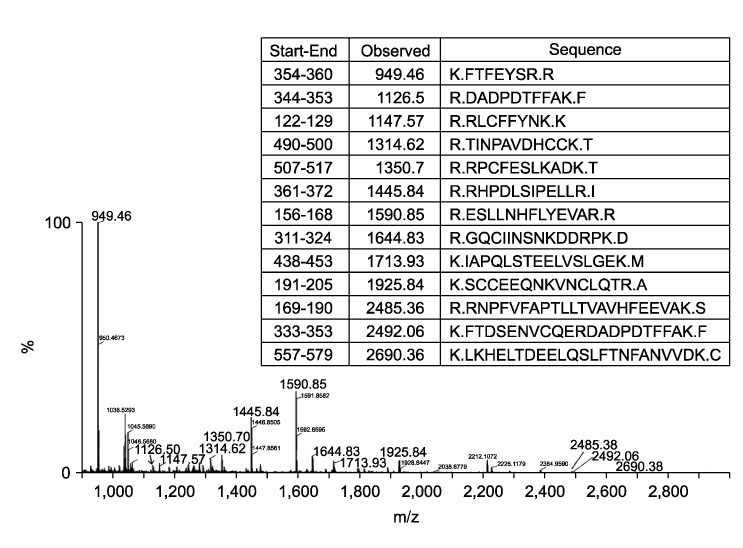
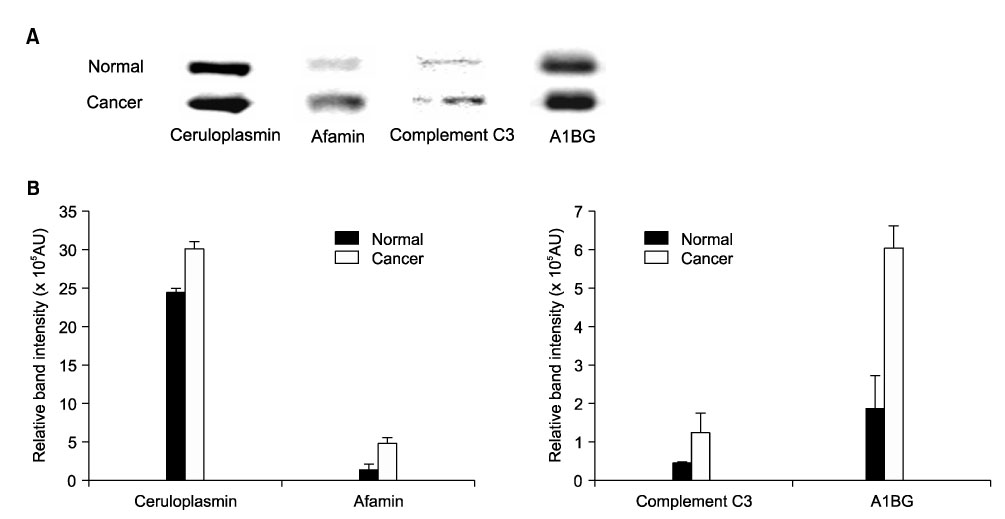
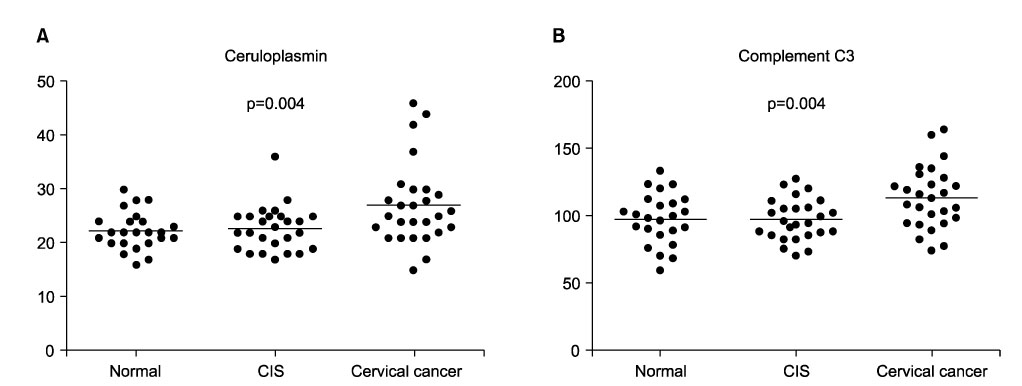
To compare plasma protein expression between patients with squamous cell carcinoma (SCC) of the cervix and normal controls. METHODS: Plasma samples from patients with benign gynecological disease (normal cervix, n=6) and cervical cancer (SCC, n=6) were subjected to plasma proteomic analysis using two dimensional gel electrophoresis (2-DE) and matrix-assisted laser desorption/ionization mass spectroscopy (MALDI-MS). Western blotting and immunoturbidimetric assay were performed to validate the results of 2-DE. RESULTS: Eight proteins showed differential expression between controls and SCC patients; six (ceruloplasmin, complement C3, afamin precursor, alpha-1-B-glycoprotein, transferrin, alpha-fibrinogen precursor) were up-regulated, while two (chain A, crystal structure of antithrombin and apolipoprotein A-IV precursor) were down-regulated in the plasma of SCC patients. Western blotting analysis revealed significant elevation of ceruloplasmin, complement C3, afamin, and alpha-1-B-glycoprotein in the plasma of SCC patients in comparison to controls. Immunoturbidimetric assay of a larger group confirmed the results of 2-DE and Western blotting, and showed that ceruloplasmin and complement C3 were significantly elevated in the plasma of SCC patients in comparison with controls and patients with carcinoma in situ (CIS) of the uterine cervix. CONCLUSION: Plasma protein expression determined using 2-DE and MALDI-MS will give a chance to identify tumor-specific biomarkers for SCC of the cervix.
Keyword
MeSH Terms
-
Apolipoproteins
Apolipoproteins A
Biomarkers
Blood Proteins
Blotting, Western
Carcinoma in Situ
Carcinoma, Squamous Cell
Ceruloplasmin
Cervix Uteri
Complement C3
Electrophoresis, Gel, Two-Dimensional
Female
Humans
Mass Spectrometry
Plasma
Proteins
Spectrometry, Mass, Matrix-Assisted Laser Desorption-Ionization
Transferrin
Uterine Cervical Neoplasms
Apolipoproteins
Apolipoproteins A
Blood Proteins
Ceruloplasmin
Complement C3
Proteins
Transferrin
Figure
Reference
-
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.2. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. 2008. Fort Washington: National Comprehensive Cancer Network.3. Liao SY, Stanbridge EJ. Expression of MN/CA9 protein in Papanicolaou smears containing atypical glandular cells of undetermined significance is a diagnostic biomarker of cervical dysplasia and neoplasia. Cancer. 2000. 88:1108–1121.4. Kurman RJ, Henson DE, Herbst AL, Noller KL, Schiffman MH. Interim guidelines for management of abnormal cervical cytology: The 1992 National Cancer Institute Workshop. JAMA. 1994. 271:1866–1869.5. Koss LG. Cervical (Pap) smear: New directions. Cancer. 1993. 71:4 Suppl. 1406–1412.6. Lin YW, Lai HC, Lin CY, Chiou JY, Shui HA, Chang CC, et al. Plasma proteomic profiling for detecting and differentiating in situ and invasive carcinomas of the uterine cervix. Int J Gynecol Cancer. 2006. 16:1216–1224.7. Shen Y, Kim J, Strittmatter EF, Jacobs JM, Camp DG 2nd, Fang R, et al. Characterization of the human blood plasma proteome. Proteomics. 2005. 5:4034–4045.8. Shoshan SH, Admon A. Proteomics in clinical laboratory diagnosis. Adv Clin Chem. 2005. 39:159–184.9. Joo WA, Lee DY, Kim CW. Development of an effective sample preparation method for the proteome analysis of body fluids using 2-D gel electrophoresis. Biosci Biotechnol Biochem. 2003. 67:1574–1577.10. Anderson NL, Polanski M, Pieper R, Gatlin T, Tirumalai RS, Conrads TP, et al. The human plasma proteome: A non-redundant list developed by combination of four separate sources. Mol Cell Proteomics. 2004. 3:311–326.11. Jacobs JM, Adkins JN, Qian WJ, Liu T, Shen Y, Camp DG 2nd, et al. Utilizing human blood plasma for proteomic biomarker discovery. J Proteome Res. 2005. 4:1073–1085.12. Doustjalali SR, Yusof R, Govindasamy GK, Bustam AZ, Pillay B, Hashim OH. Patients with nasopharyngeal carcinoma demonstrate enhanced serum and tissue ceruloplasmin expression. J Med Invest. 2006. 53:20–28.13. Liu W, Liu B, Xin L, Zhang Y, Chen X, Zhu Z, et al. Down-regulated expression of complement factor I: a potential suppressive protein for gastric cancer identified by serum proteome analysis. Clin Chim Acta. 2007. 377:119–126.14. Feng JT, Liu YK, Song HY, Dai Z, Qin LX, Almofti MR, et al. Heat-shock protein 27: A potential biomarker for hepatocellular carcinoma identified by serum proteome analysis. Proteomics. 2005. 5:4581–4588.15. Wilkins MR, Pasquali C, Appel RD, Ou K, Golaz O, Sanchez JC. From proteins to proteomes: Large-scale protein identification by two-dimensional electrophoresis and amino acid analysis. Biotechnology. 1996. 14:61–65.16. Rosenfeld J, Capdevielle J, Guillemot JC, Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem. 1992. 203:173–179.17. Lowndes SA, Harris AL. The role of copper in tumour angiogenesis. J Mammary Gland Biol Neoplasia. 2005. 10:299–310.18. Martin F, Linden T, Katschinski DM, Oehme F, Flamme I, Mukhopadhyay CK, et al. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: Implications for ceruloplasmin regulation. Blood. 2005. 105:4613–4619.19. Nasulewicz A, Mazur A, Opolski A. Role of copper in tumour angiogenesis: Clinical implications. J Trace Elem Med Biol. 2004. 18:1–8.20. Pejovic M, Djordjevic V, Ignjatovic I, Stamenic T, Stefanovic V. Serum levels of some acute phase proteins in kidney and urinary tract urothelial cancers. Int Urol Nephrol. 1997. 29:427–432.21. Agroyannis B, Dalamangas A, Dardouphas K, Fortoynas C, Saloum G, Stringou E, et al. Serum transferrin and ceruloplasmin in patients with cancer of the gastrointestinal and other systems. Anticancer Res. 1994. 14:2201–2203.22. Ros-Bullón MR, Sánchez-Pedreño P, Martínez-Liarte JH. Serum ceruloplasmin in melanoma patients. Anticancer Res. 2001. 21:629–632.23. Jurianz K, Ziegler S, Garcia-Schüler H, Kraus S, Bohana-Kashtan O, Fishelson Z, et al. Complement resistance of tumor cells: Basal and induced mechanisms. Mol Immunol. 1999. 36:929–939.24. Sahu A, Lambris JD. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol Rev. 2001. 180:35–48.25. Frade R. Structure and functions of proteases which cleave human C3 and are expressed on normal or tumor human cells: Some are involved in tumorigenic and metastatic properties of human melanoma cells. Immunopharmacology. 1999. 42:39–45.26. Lucas SD, Karlsson-Parra A, Nilsson B, Grimelius L, Akerstrom G, Rastad J, et al. Tumor-specific deposition of immunoglobulin G and complement in papillary thyroid carcinoma. Hum Pathol. 1996. 27:1329–1335.27. Niculescu F, Rus HG, Retegan M, Vlaicu R. Persistent complement activation on tumor cells in breast cancer. Am J Pathol. 1992. 140:1039–1043.28. Yamakawa M, Yamada K, Tsuge T, Ohrui H, Ogata T, Dobashi M, et al. Protection of thyroid cancer cells by complement-regulatory factors. Cancer. 1994. 73:2808–2817.29. Bernet-Camard MF, Coconnier MH, Hudault S, Servin AL. Differential expression of complement proteins and regulatory decay accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut. 1996. 38:248–253.30. Niehans GA, Cherwitz DL, Staley NA, Knapp DJ, Dalmasso AP. Human carcinomas variably express the complement inhibitory proteins CD46 (membrane cofactor protein), CD55 (decay-accelerating factor), and CD59 (protectin). Am J Pathol. 1996. 149:129–142.31. Hanas JS, Hocker JR, Cheung JY, Larabee JL, Lerner MR, Lightfoot SA, et al. Biomarker identification in human pancreatic cancer sera. Pancreas. 2008. 36:61–69.32. Lee IN, Chen CH, Sheu JC, Lee HS, Huang GT, Chen DS, et al. Identification of complement C3a as a candidate biomarker in human chronic hepatitis C and HCV-related hepatocellular carcinoma using a proteomics approach. Proteomics. 2006. 6:2865–2873.33. Bjørge L, Hakulinen J, Vintermyr OK, Jarva H, Jensen TS, Iversen OE, et al. Ascitic complement system in ovarian cancer. Br J Cancer. 2005. 92:895–905.34. Bélanger L, Roy S, Allard D. New albumin gene 3' adjacent to the alpha 1-fetoprotein locus. J Biol Chem. 1994. 269:5481–5484.35. Lichenstein HS, Lyons DE, Wurfel MM, Johnson DA, McGinley MD, Leidli JC, et al. Afamin is a new member of the albumin, alpha-fetoprotein, and vitamin D-binding protein gene family. J Biol Chem. 1994. 269:18149–18154.36. Jerkovic L, Voegele AF, Chwatal S, Kronenberg F, Radcliffe CM, Wormald MR, et al. Afamin is a novel human vitamin E-binding glycoprotein characterization and in vitro expression. J Proteome Res. 2005. 4:889–899.37. Wu GX, Lin YM, Zhou TH, Gao H, Pei G. Significant down-regulation of alpha-albumin in human hepatoma and its implication. Cancer Lett. 2000. 160:229–236.38. Jackson D, Craven RA, Hutson RC, Graze I, Lueth P, Tonge RP, et al. Proteomic profiling identifies afamin as a potential biomarker for ovarian cancer. Clin Cancer Res. 2007. 13:7370–7379.39. Ishioka N, Takahashi N, Putnam FW. Amino acid sequence of human plasma alpha 1B-glycoprotein: Homology to the immunoglobulin supergene family. Proc Natl Acad Sci U S A. 1986. 83:2363–2367.40. Abdul-Rahman PS, Lim BK, Hashim OH. Expression of high-abundance proteins in sera of patients with endometrial and cervical cancers: analysis using 2-DE with silver staining and lectin detection methods. Electrophoresis. 2007. 28:1989–1996.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Lymphoepitheliomalike Carcinoma of the Uterine Cervix
- Lectins Binding in Squamous Cell Carcinoma of the Uterine Cervix as a Diagnostic and Prognostic Marker
- Sarcomatoid squamous cell carcinoma of uterine cervix
- A Case of Ovarian Squamous Cell Carcinoma in a Patient with Microinvasive Squamous Cell Carcinoma of Cervix
- A case of neutrophilia related to a cytokine-producing relapsed squamous cell carcinoma of the uterine cervix arising from the rectovaginal septum