Korean J Physiol Pharmacol.
2010 Dec;14(6):377-384. 10.4196/kjpp.2010.14.6.377.
Metformin Inhibits Isoproterenol-induced Cardiac Hypertrophy in Mice
- Affiliations
-
- 1Department of Physiology, College of Medicine, Yeungnam University, Daegu 705-717, Korea. sypark@med.yu.ac.kr
- 2Aging-associated Vascular Disease Research Center, College of Medicine, Yeungnam University, Daegu 705-717, Korea.
- 3Department of Internal Medicine, Dankook University College of Medicine, Cheonan 330-715, Korea.
- 4Department of Orthopedics, College of Medicine, Yeungnam University, Daegu 705-717, Korea.
- KMID: 2071709
- DOI: http://doi.org/10.4196/kjpp.2010.14.6.377
Abstract
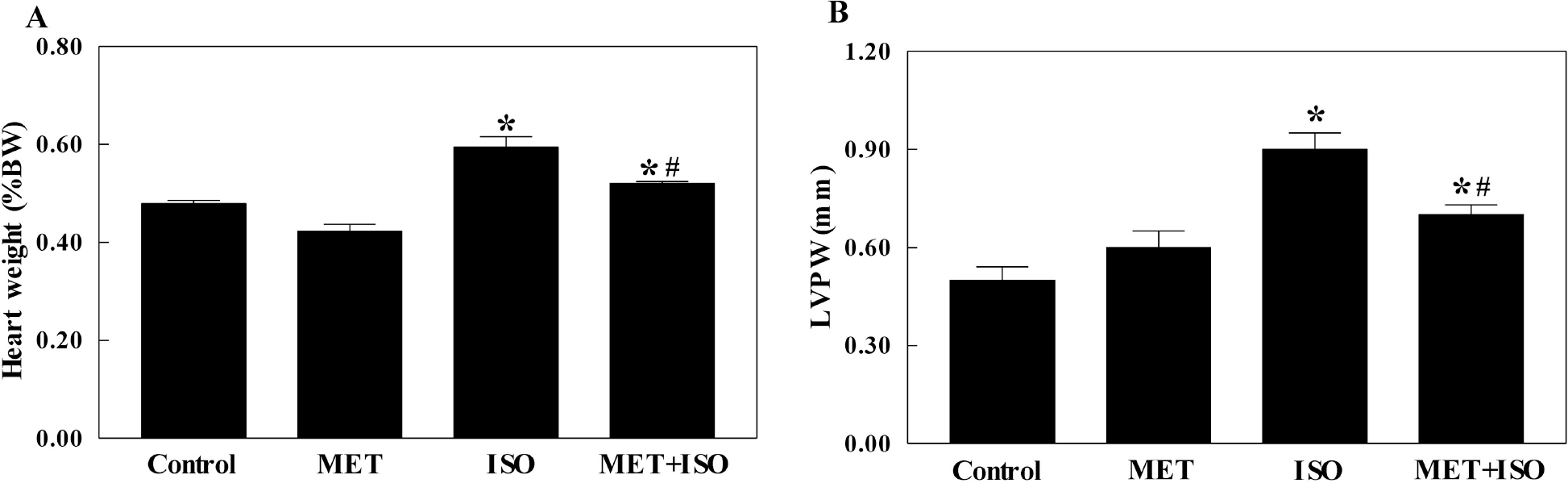
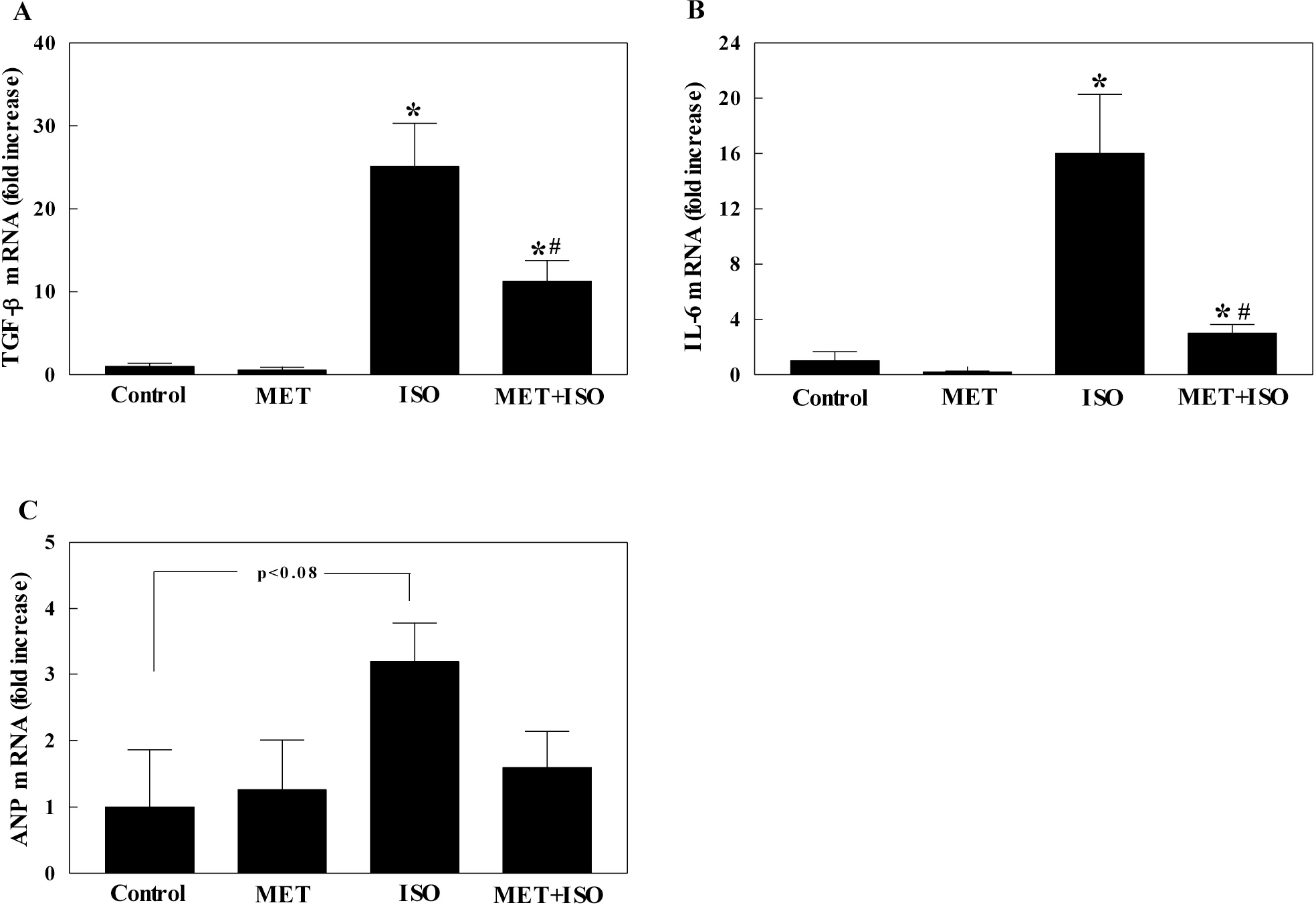
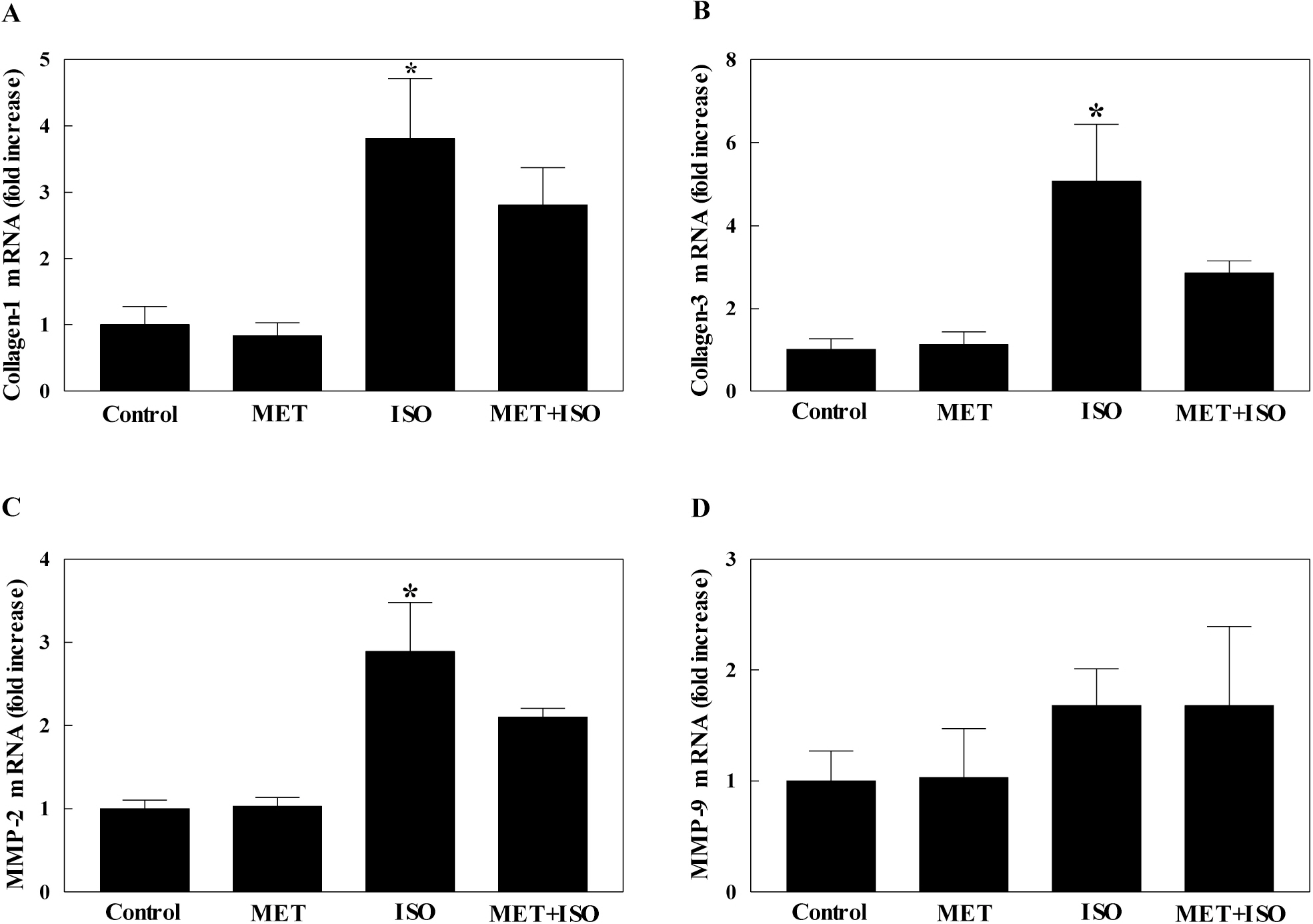
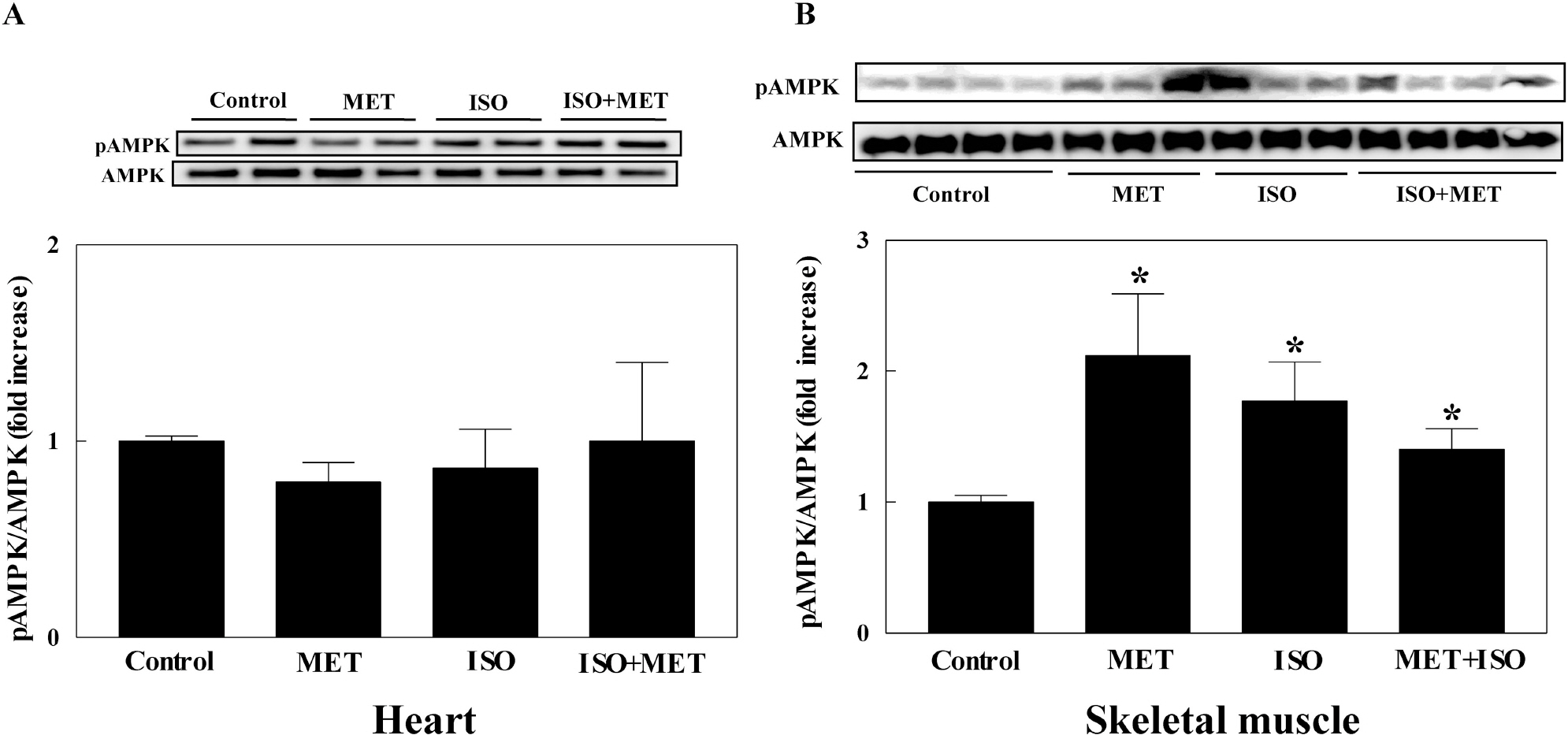
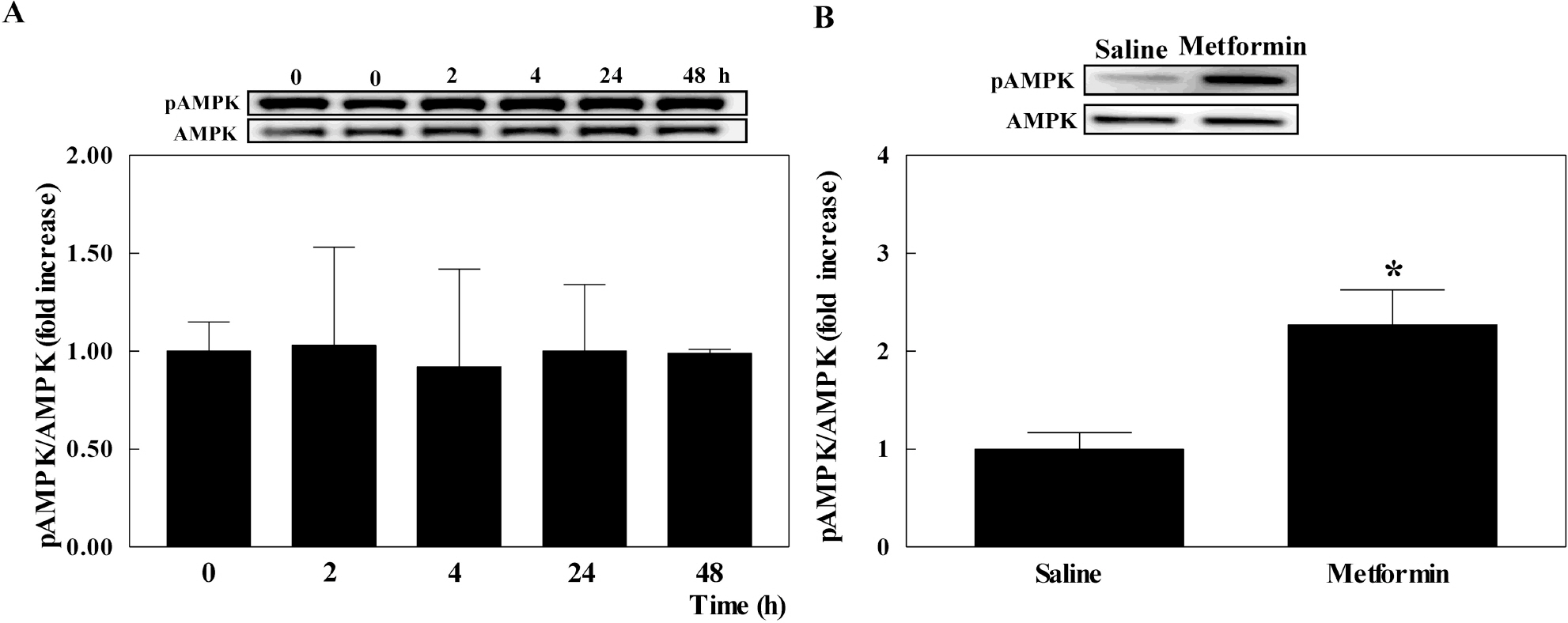
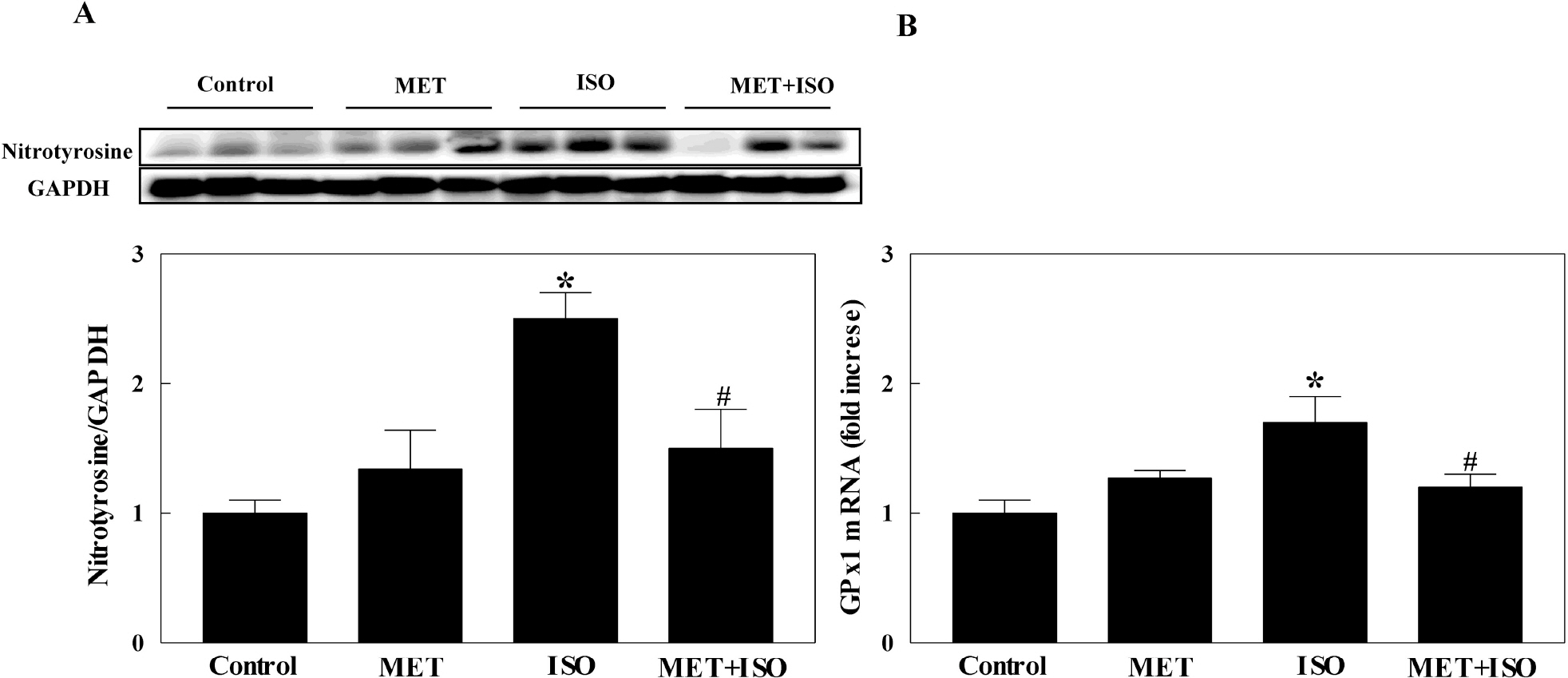
- The present study examined whether metformin treatment prevents isoporterenol-induced cardiac hypertrophy in mice. Chronic subcutaneous infusion of isoproterenol (15 mg/kg/24 h) for 1 week using an osmotic minipump induced cardiac hypertrophy measured by the heart-to-body weight ratio and left ventricular posterior wall thickness. Cardiac hypertrophy was accompanied with increased interleukin-6 (IL-6), transforming growth factor (TGF)-beta, atrial natriuretic peptide (ANP), collagen I and III, and matrix metallopeptidase 2 (MMP-2). Coinfusion of metformin (150 mg/kg/24 h) with isoproterenol partially inhibited cardiac hypertrophy that was followed by reduced IL-6, TGF-beta, ANP, collagen I and III, and MMP-2. Chronic subcutaneous infusion of metformin did not increase AMP-activated protein kinase (AMPK) activity in heart, although acute intraperitoneal injection of metformin (10 mg/kg) increased AMPK activity. Isoproterenol increased nitrotyrosine levels and mRNA expression of antioxidant enzyme glutathione peroxidase and metformin treatment normalized these changes. These results suggest that metformin inhibits cardiac hypertrophy through attenuating oxidative stress.
Keyword
MeSH Terms
-
AMP-Activated Protein Kinases
Animals
Atrial Natriuretic Factor
Cardiomegaly
Collagen
Glutathione Peroxidase
Heart
Infusions, Subcutaneous
Injections, Intraperitoneal
Interleukin-6
Isoproterenol
Metformin
Mice
Oxidative Stress
RNA, Messenger
Transforming Growth Factor beta
Transforming Growth Factors
Tyrosine
AMP-Activated Protein Kinases
Atrial Natriuretic Factor
Collagen
Glutathione Peroxidase
Interleukin-6
Isoproterenol
Metformin
RNA, Messenger
Transforming Growth Factor beta
Transforming Growth Factors
Tyrosine
Figure
Cited by 3 articles
-
MicroRNA-1 in Cardiac Diseases and Cancers
Jianzhe Li, Xiaomin Dong, Zhongping Wang, Jianhua Wu
Korean J Physiol Pharmacol. 2014;18(5):359-363. doi: 10.4196/kjpp.2014.18.5.359.EGCG Blocked Phenylephrin-Induced Hypertrophy in H9C2 Cardiomyocytes, by Activating AMPK-Dependent Pathway
Yi Cai, Li Zhao, Yuan Qin, Xiao-Qian Wu
Korean J Physiol Pharmacol. 2015;19(3):203-210. doi: 10.4196/kjpp.2015.19.3.203.Geraniin attenuates isoproterenol-induced cardiac hypertrophy by inhibiting inflammation, oxidative stress and cellular apoptosis
Jiaqi Ding, Shenjie Zhang, Qi Li, Boyu Xia, Jingjing Wu, Xu Lu, Chao Huang, Xiaomei Yuan, Qingsheng You
Korean J Physiol Pharmacol. 2025;29(3):307-319. doi: 10.4196/kjpp.24.200.
Reference
-
References
1. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990; 322:1561–1566.
Article2. Barry SP, Davidson SM, Townsend PA. Molecular regulation of cardiac hypertrophy. Int J Biochem Cell Biol. 2008; 40:2023–2039.
Article3. Corea L, Bentivoglio M, Verdecchia P. Echocardiographic left ventricular hypertrophy as related to arterial pressure and plasma norepinephrine concentration in arterial hypertension. Reversal by atenolol treatment. Hypertension. 1983; 5:837–843.
Article4. Greenwood JP, Scott EM, Stoker JB, Mary DA. Hypertensive left ventricular hypertrophy: relation to peripheral sympathetic drive. J Am Coll Cardiol. 2001; 38:1711–1717.
Article5. Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003; 108:560–565.
Article6. Cha HN, Hong GR, Kim YW, Kim JY, Dan JM, Park SY. Deficiency of iNOS Does Not Prevent Isoproterenol-induced Cardiac Hypertrophy in Mice. Korean J Physiol Pharmacol. 2009; 13:153–159.
Article7. Shantz LM, Feith DJ, Pegg AE. Targeted overexpression of ornithine decarboxylase enhances beta-adrenergic agonist-induced cardiac hypertrophy. Biochem J. 2001; 358:25–32.8. Osadchii OE. Cardiac hypertrophy induced by sustained beta-adrenoreceptor activation: pathophysiological aspects. Heart Fail Rev. 2007; 12:66–86.9. Krenek P, Kmecova J, Kucerova D, Bajuszova Z, Musil P, Gazova A, Ochodnicky P, Klimas J, Kyselovic J. Isoproterenol-induced heart failure in the rat is associated with nitric oxide-dependent functional alterations of cardiac function. Eur J Heart Fail. 2009; 11:140–146.
Article10. Szabo J, Csaky L, Szegi J. Experimental cardiac hypertrophy induced by isoproterenol in the rat. Acta Physiol Acad Sci Hung. 1975; 46:281–285.11. Abbasi F, Chu JW, McLaughlin T, Lamendola C, Leary ET, Reaven GM. Effect of metformin treatment on multiple cardiovascular disease risk factors in patients with type 2 diabetes mellitus. Metabolism. 2004; 53:159–164.
Article12. Rossetti L, DeFronzo RA, Gherzi R, Stein P, Andraghetti G, Falzetti G, Shulman GI, Klein-Robbenhaar E, Cordera R. Effect of metformin treatment on insulin action in diabetic rats: in vivo and in vitro correlations. Metabolism. 1990; 39:425–435.13. Stafford JM, Elasy T. Treatment update: thiazolidinediones in combination with metformin for the treatment of type 2 diabetes. Vasc Health Risk Manag. 2007; 3:503–510.14. Perriello G, Misericordia P, Volpi E, Santucci A, Santucci C, Ferrannini E, Ventura MM, Santeusanio F, Brunetti P, Bolli GB. Acute antihyperglycemic mechanisms of metformin in NIDDM. Evidence for suppression of lipid oxidation and hepatic glucose production. Diabetes. 1994; 43:920–928.
Article15. Johnson JA, Majumdar SR, Simpson SH, Toth EL. Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care. 2002; 25:2244–2248.
Article16. Johnson JA, Simpson SH, Toth EL, Majumdar SR. Reduced cardiovascular morbidity and mortality associated with metformin use in subjects with Type 2 diabetes. Diabet Med. 2005; 22:497–502.
Article17. Kao J, Tobis J, McClelland RL, Heaton MR, Davis BR, Holmes DR Jr, Currier JW. Relation of metformin treatment to clinical events in diabetic patients undergoing percutaneous intervention. Am J Cardiol. 2004; 93:1347–1350. A1345.
Article18. Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006; 55:496–505.19. Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009; 104:403–411.
Article20. Masson S, Arosio B, Luvara G, Gagliano N, Fiordaliso F, Santambrogio D, Vergani C, Latini R, Annoni G. Remodelling of cardiac extracellular matrix during beta-adrenergic stimulation: upregulation of SPARC in the myocardium of adult rats. J Mol Cell Cardiol. 1998; 30:1505–1514.21. Hori Y, Kunihiro S, Sato S, Yoshioka K, Hara Y, Kanai K, Hoshi F, Itoh N, Higuchi S. Doxycycline attenuates isoproterenol-induced myocardial fibrosis and matrix metalloproteinase activity in rats. Biol Pharm Bull. 2009; 32:1678–1682.
Article22. Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004; 279:32771–32779.
Article23. Mikaelian I, Coluccio D, Morgan KT, Johnson T, Ryan AL, Rasmussen E, Nicklaus R, Kanwal C, Hilton H, Frank K, Fritzky L, Wheeldon EB. Temporal gene expression profiling indicates early up-regulation of interleukin-6 in isoproterenol-induced myocardial necrosis in rat. Toxicol Pathol. 2008; 36:256–264.
Article24. Tanaka T, Kanda T, Itoh T, Tsugawa H, Takekoshi N, Yamakawa J, Kurimoto M, Kurabayashi M. Increased cardiac weight in interleukin-6 transgenic mice with viral infection accompanies impaired expression of natriuretic peptide genes. Res Commun Mol Pathol Pharmacol. 2001; 110:275–283.25. Villarreal FJ, Dillmann WH. Cardiac hypertrophy-induced changes in mRNA levels for TGF-beta 1, fibronectin, and collagen. Am J Physiol. 1992; 262:H1861–1866.
Article26. Li RK, Li G, Mickle DA, Weisel RD, Merante F, Luss H, Rao V, Christakis GT, Williams WG. Overexpression of transforming growth factor-beta1 and insulin-like growth factor-I in patients with idiopathic hypertrophic cardiomyopathy. Circulation. 1997; 96:874–881.27. Schmechel A, Grimm M, El-Armouche A, Hoppner G, Schwoerer AP, Ehmke H, Eschenhagen T. Treatment with atorvastatin partially protects the rat heart from harmful catecholamine effects. Cardiovasc Res. 2009; 82:100–106.
Article28. Briest W, Rassler B, Deten A, Zimmer HG. Norepinephrine-induced cardiac hypertrophy and fibrosis are not due to mast cell degranulation. Mol Cell Biochem. 2003; 252:229–237.
Article29. Yamada T, Nagata K, Cheng XW, Obata K, Saka M, Miyachi M, Naruse K, Nishizawa T, Noda A, Izawa H, Kuzuya M, Okumura K, Murohara T, Yokota M. Long-term administration of nifedipine attenuates cardiac remodeling and diastolic heart failure in hypertensive rats. Eur J Pharmacol. 2009; 615:163–170.
Article30. Lin Y, Wang LN, Xi YH, Li HZ, Xiao FG, Zhao YJ, Tian Y, Yang BF, Xu CQ. L-arginine inhibits isoproterenol-induced cardiac hypertrophy through nitric oxide and polyamine pathways. Basic Clin Pharmacol Toxicol. 2008; 103:124–130.
Article31. Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circ Res. 1996; 79:363–380.
Article32. Torres SH, De Sanctis JB, de L Briceño M, Hernandez N, Finol HJ. Inflammation and nitric oxide production in skeletal muscle of type 2 diabetic patients. J Endocrinol. 2004; 181:419–427.
Article33. Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996; 271:C1424–1437.
Article34. Nadruz W Jr, Lagosta VJ, Moreno H Jr, Coelho OR, Franchini KG. Simvastatin prevents load-induced protein tyrosine nitration in overloaded hearts. Hypertension. 2004; 43:1060–1066.
Article35. Balta N, Stoian I, Petec C, Petec G. Decreased SOD activity and increased nitrates level in rat heart with left ventricular hypertrophy induced by isoproterenol. Rom J Physiol. 1999; 36:175–182.36. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001; 108:1167–1174.
Article37. Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, Takashima S, Sanada S, Sugimachi M, Komamura K, Mochizuki N, Kitakaze M. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 2009; 119:2568–2577.38. Solskov L, Lofgren B, Kristiansen SB, Jessen N, Pold R, Nielsen TT, Botker HE, Schmitz O, Lund S. Metformin induces cardioprotection against ischaemia/reperfusion injury in the rat heart 24 hours after administration. Basic Clin Pharmacol Toxicol. 2008; 103:82–87.
Article39. Hou X, Song J, Li XN, Zhang L, Wang X, Chen L, Shen YH. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun. 2010; 396:199–205.
Article40. Saeedi R, Parsons HL, Wambolt RB, Paulson K, Sharma V, Dyck JR, Brownsey RW, Allard MF. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am J Physiol Heart Circ Physiol. 2008; 294:H2497–2506.
Article41. Bergheim I, Guo L, Davis MA, Lambert JC, Beier JI, Duveau I, Luyendyk JP, Roth RA, Arteel GE. Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1. Gastroenterology. 2006; 130:2099–2112.
Article42. Elia E, Sander V, Luchetti CG, Solano ME, Di Girolamo G, Gonzalez C, Motta AB. The mechanisms involved in the action of metformin in regulating ovarian function in hyperandrogenized mice. Mol Hum Reprod. 2006; 12:475–481.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The alteration of Ca2+-activated K+ channels in coronary arterial smooth muscle cells isolated from isoproterenol-induced cardiac hypertrophy in rabbit
- Deficiency of iNOS Does Not Prevent Isoproterenol-induced Cardiac Hypertrophy in Mice
- Altered delayed rectifier K+ current of rabbit coronary arterial myocytes in isoproterenol-induced hypertrophy
- Differential Activation of Ras/Raf/MAPK Pathway between Heart and Cerebral Artery in Isoproterenol-induced Cardiac Hypertrophy
- Expression of natriuretic peptide mRNAs in isoproterenol-induced cardiac hypertrophy in rats.