Lab Anim Res.
2015 Jun;31(2):69-77. 10.5625/lar.2015.31.2.69.
Prolonged oral administration of Gastrodia elata extract improves spatial learning and memory of scopolamine-treated rats
- Affiliations
-
- 1Huvet Co. Ltd., Iksan, Korea. leeapf@nate.com
- 2Animal Disease Research Unit, Wonkwang University, Iksan, Korea.
- 3Center for Animal Resources Development, Wonkwang University, Iksan, Korea.
- 4Namyoung Pharm, Muju, Korea.
- 5Research Center for Industrial Development of Biofood Materials, Chonbuk National University, Jeonju, Korea. j.oh@jbnu.ac.kr
- 6Department of Food Science and Technology, Chonbuk National University, Jeonju, Korea.
- KMID: 2053680
- DOI: http://doi.org/10.5625/lar.2015.31.2.69
Abstract
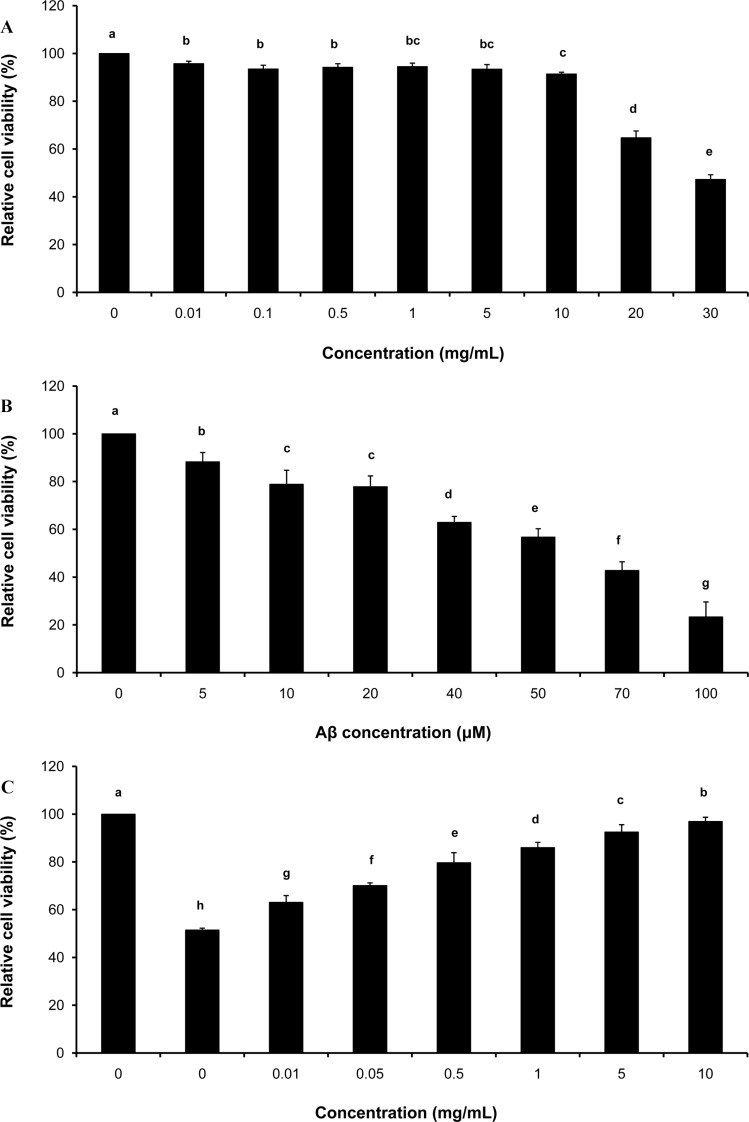
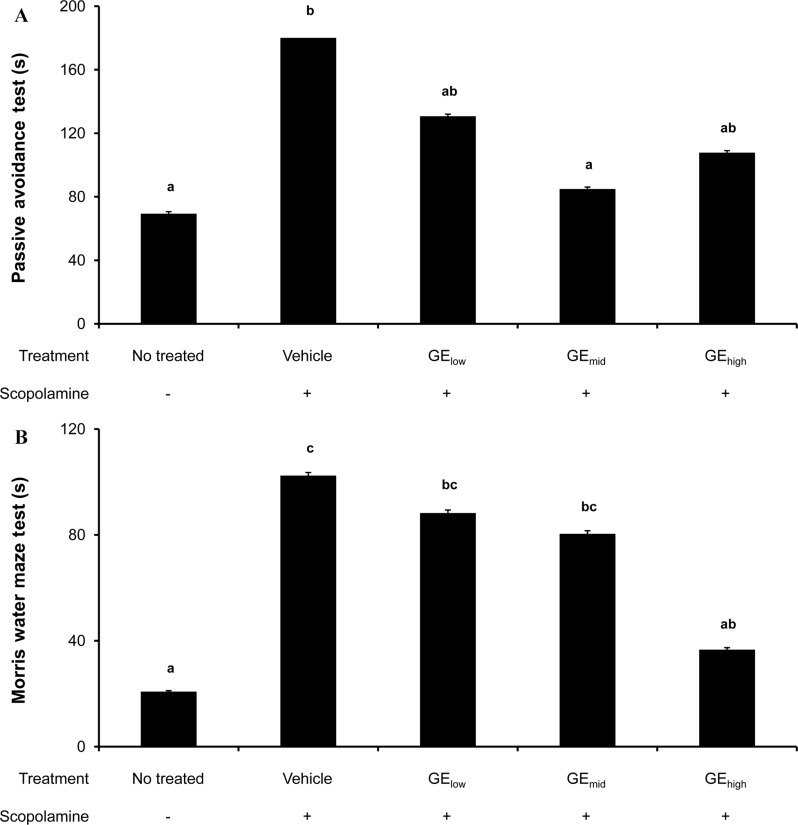
- Gastrodia elata (GE) is traditionally used for treatment of various disorders including neurodegenerative diseases such as Alzheimer's disease. To investigate the neuroprotective effect of GE, amyloid-beta peptide (Abeta)-treated PC12 cells were cultured with GE aqueous extract. In vitro assay demonstrated that 50 microM of pre-aggregated Abeta was lethal to about a half portion of PC12 cells and that Abeta aggregate-induced cell death was significantly decreased with GE treatment at < or =10 mg/mL in a dose-dependent manner. To further examine in vivo cognitive-improving effects, an artificial amnesic animal model, scopolamine-injected Sprague-Dawley rats, were orally administered the extract for 6 weeks followed by behavioral tests (the passive avoidance test and Morris water maze test). The results showed that an acute treatment with scopolamine (1 mg/kg of body weight) effectively induced memory impairment in normal rats and that the learning and memory capability of scopolamine-treated rats improved after prolonged administration of GE extract (50, 250 and 500 mg/kg of body weight for 6 weeks). These findings suggest that a GE regimen may potentially ameliorate learning and memory deficits and/or cognitive impairments caused by neuronal cell death.
Keyword
MeSH Terms
Figure
Reference
-
1. Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010; 362(4):329–344. PMID: 20107219.
Article2. Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005; 120(4):545–555. PMID: 15734686.
Article3. Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer's disease. J Neurosci. 2006; 26(35):9047–9056. PMID: 16943563.
Article4. Ojemann LM, Nelson WL, Shin DS, Rowe AO, Buchanan RA. Tian ma, an ancient Chinese herb, offers new options for the treatment of epilepsy and other conditions. Epilepsy Behav. 2006; 8(2):376–383. PMID: 16461011.
Article5. Hsieh CL, Tang NY, Chiang SY, Hsieh CT, Lin JG. Anticonvulsive and free radical scavenging actions of two herbs, Uncaria rhynchophylla (MIQ) Jack and Gastrodia elata Bl., in kainic acid-treated rats. Life Sci. 1999; 65(20):2071–2082. PMID: 10579461.
Article6. Zhu L, Guan H, Cui C, Tian S, Yang D, Wang X, Zhang S, Wang L, Jiang H. Gastrodin inhibits cell proliferation in vascular smooth muscle cells and attenuates neointima formation in vivo. Int J Mol Med. 2012; 30(5):1034–1040. PMID: 22922870.
Article7. Shu C, Chen C, Zhang DP, Guo H, Zhou H, Zong J, Bian Z, Dong X, Dai J, Zhang Y, Tang Q. Gastrodin protects against cardiac hypertrophy and fibrosis. Mol Cell Biochem. 2012; 359(1-2):9–16. PMID: 21833534.
Article8. Ha JH, Shin SM, Lee SK, Kim JS, Shin US, Huh K, Kim JA, Yong CS, Lee NJ, Lee DU. In vitro effects of hydroxybenzaldehydes from Gastrodia elata and their analogues on GABAergic neurotransmission, and a structure-activity correlation. Planta Med. 2001; 67(9):877–880. PMID: 11745032.9. Ha JH, Lee DU, Lee JT, Kim JS, Yong CS, Kim JA, Ha JS, Huh K. 4-Hydroxybenzaldehyde from Gastrodia elata B1. is active in the antioxidation and GABAergic neuromodulation of the rat brain. J Ethnopharmacol. 2000; 73(1-2):329–333. PMID: 11025174.
Article10. Mishra M, Huang J, Lee YY, Chua DS, Lin X, Hu JM, Heese K. Gastrodia elata modulates amyloid precursor protein cleavage and cognitive functions in mice. Biosci Trends. 2011; 5(3):129–138. PMID: 21788698.11. Manavalan A, Ramachandran U, Sundaramurthi H, Mishra M, Sze SK, Hu JM, Feng ZW, Heese K. Gastrodia elata Blume (tianma) mobilizes neuro-protective capacities. Int J Biochem Mol Biol. 2012; 3(2):219–241. PMID: 22773961.12. Phillips RG, LeDoux JE. Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contextual fear conditioning. J Neurosci. 1995; 15(7 Pt 2):5308–5315. PMID: 7623153.
Article13. Bayer TA, Wirths O, Majtényi K, Hartmann T, Multhaup G, Beyreuther K, Czech C. Key factors in Alzheimer's disease: beta-amyloid precursor protein processing, metabolism and intraneuronal transport. Brain Pathol. 2001; 11(1):1–11. PMID: 11145195.14. Liu P, Jing Y, Collie ND, Campbell SA, Zhang H. Pre-aggregated Aβ(25-35) alters arginine metabolism in the rat hippocampus and prefrontal cortex. Neuroscience. 2011; 193:269–282. PMID: 21807072.
Article15. Ng CF, Ko CH, Koon CM, Xian JW, Leung PC, Fung KP, Chan HY, Lau CB. The Aqueous Extract of Rhizome of Gastrodia elata Protected Drosophila and PC12 Cells against Beta-Amyloid-Induced Neurotoxicity. Evid Based Complement Alternat Med. 2013; 2013:516741. PMID: 24174977.16. Wesnes KA, Simpson PM, White L, Pinker S, Jertz G, Murphy M, Siegfried K. Cholinesterase inhibition in the scopolamine model of dementia. Ann N Y Acad Sci. 1991; 640:268–271. PMID: 1776749.
Article17. Deutsch JA. The cholinergic synapse and the site of memory. Science. 1971; 174(4011):788–794. PMID: 4330469.
Article18. Ebert U, Kirch W. Scopolamine model of dementia: electro-encephalogram findings and cognitive performance. Eur J Clin Invest. 1998; 28(11):944–949. PMID: 9824440.
Article19. Bartus RT, Dean RL 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982; 217(4558):408–414. PMID: 7046051.
Article20. Izquierdo I. Mechanism of action of scopolamine as an amnestic. Trends Pharmacol Sci. 1989; 10(5):175–177. PMID: 2667223.
Article21. Wu CR, Hsieh MT, Huang SC, Peng WH, Chang YS, Chen CF. Effects of Gastrodia elata and its active constituents on scopolamine-induced amnesia in rats. Planta Med. 1996; 62(4):317–321. PMID: 8792662.22. Hsieh MT, Wu CR, Chen CF. Gastrodin and p-hydroxybenzyl alcohol facilitate memory consolidation and retrieval, but not acquisition, on the passive avoidance task in rats. J Ethnopharmacol. 1997; 56(1):45–54. PMID: 9147253.
Article23. Kim HJ, Moon KD, Oh SY, Kim SP, Lee SR. Ether fraction of methanol extracts of Gastrodia elata, a traditional medicinal herb, protects against kainic acid-induced neuronal damage in the mouse hippocampus. Neurosci Lett. 2001; 314(1-2):65–68. PMID: 11698148.
Article24. An SJ, Park SK, Hwang IK, Choi SY, Kim SK, Kwon OS, Jung SJ, Baek NI, Lee HY, Won MH, Kang TC. Gastrodin decreases immunoreactivities of gamma-aminobutyric acid shunt enzymes in the hippocampus of seizure-sensitive gerbils. J Neurosci Res. 2003; 71(4):534–543. PMID: 12548709.25. Zeng X, Zhang S, Zhang L, Zhang K, Zheng X. A study of the neuroprotective effect of the phenolic glucoside gastrodin during cerebral ischemia in vivo and in vitro. Planta Med. 2006; 72(15):1359–1365. PMID: 17089323.26. Dai JN, Zong Y, Zhong LM, Li YM, Zhang W, Bian LG, Ai QL, Liu YD, Sun J, Lu D. Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways. PLoS One. 2011; 6(7):e21891. PMID: 21765922.
Article27. Zhao X, Zou Y, Xu H, Fan L, Guo H, Li X, Li G, Zhang X, Dong M. Gastrodin protect primary cultured rat hippocampal neurons against amyloid-beta peptide-induced neurotoxicity via ERK1/2-Nrf2 pathway. Brain Res. 2012; 1482:13–21. PMID: 22982592.
Article28. Kumar H, Kim IS, More SV, Kim BW, Bahk YY, Choi DK. Gastrodin protects apoptotic dopaminergic neurons in a toxininduced Parkinson's disease model. Evid Based Complement Alternat Med. 2013; 2013:514095. PMID: 23533492.
Article29. Wang Q, Chen G, Zeng S. Distribution and metabolism of gastrodin in rat brain. J Pharm Biomed Anal. 2008; 46(2):399–404. PMID: 18053670.
Article30. Wang Q, Chen G, Zeng S. Pharmacokinetics of Gastrodin in rat plasma and CSF after i.n. and i.v. Int J Pharm. 2007; 341(1-2):20–25. PMID: 17482780.
Article31. Lin LC, Chen YF, Tsai TR, Tsai TH. Analysis of brain distribution and biliary excretion of a nutrient supplement, gastrodin, in rat. Anal Chim Acta. 2007; 590(2):173–179. PMID: 17448342.
Article32. Lin LC, Chen YF, Lee WC, Wu YT, Tsai TH. Pharmacokinetics of gastrodin and its metabolite p-hydroxybenzyl alcohol in rat blood, brain and bile by microdialysis coupled to LC-MS/MS. J Pharm Biomed Anal. 2008; 48(3):909–917. PMID: 18757149.
Article33. Zheng Q, Yue PF, Wu B, Hu PY, Wu ZF, Yang M. Pharmacokinetics comparative study of a novel Chinese traditional herbal formula and its compatibility. J Ethnopharmacol. 2011; 137(1):221–225. PMID: 21605650.
Article34. Jiang L, Dai J, Huang Z, Du Q, Lin J, Wang Y. Simultaneous determination of gastrodin and puerarin in rat plasma by HPLC and the application to their interaction on pharmacokinetics. J Chromatogr B Analyt Technol Biomed Life Sci. 2013; 915-916:8–12.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gastrodia elata Blume Attenuates 2, 4-Dinitrochlorobenzene-induced Atopic Dermatitis-like Skin Lesions in Balb/c Mice and SD Rats
- Effects of Chronic and Acute Lithium Treatment on the Long-term Potentiation and Spatial Memory in Adult Rats
- Seed Germination of Gastrodia elata Using Symbiotic Fungi, Mycena osmundicola
- Physiological Characteristics of Symbiotic Fungi Associated with the Seed Germination of Gastrodia elata
- Mycena subpiligera sp. nov., a Symbiotic Species from China Associated with the Seed Germination of Gastrodia elata