Korean J Physiol Pharmacol.
2012 Aug;16(4):243-247. 10.4196/kjpp.2012.16.4.243.
Morphologic Evidence of Anti-Tumor Specificity of T Cells Activated by Denritic Cells Derived from Peripheral Blood Mononuclear Cells of Thyroid Cancer Patients
- Affiliations
-
- 1Department of Pharmacology, Kosin University College of Medicine, Busan 602-703, Korea. dhlee@ns.kosinmed.or.kr
- KMID: 2011158
- DOI: http://doi.org/10.4196/kjpp.2012.16.4.243
Abstract
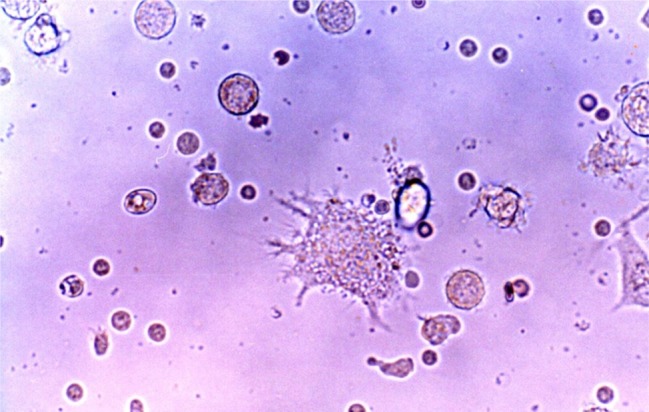
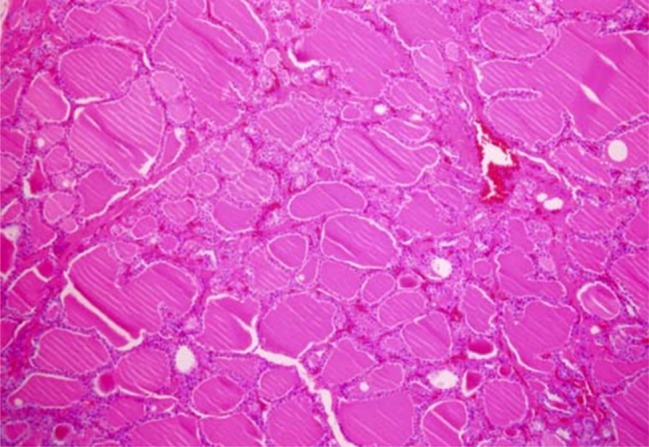
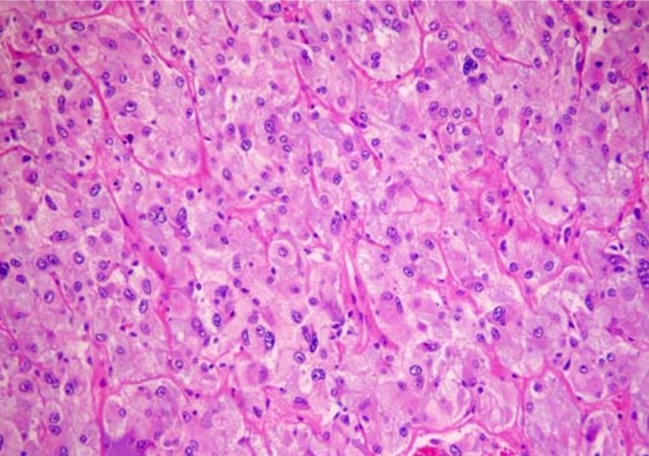
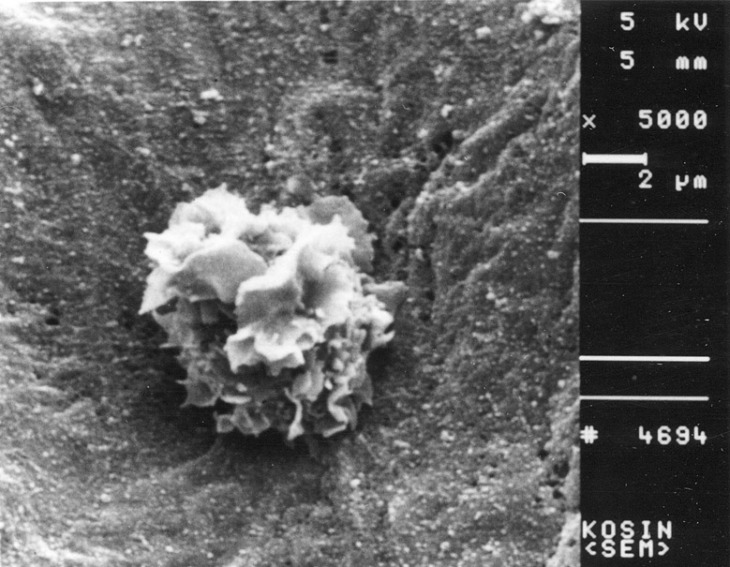
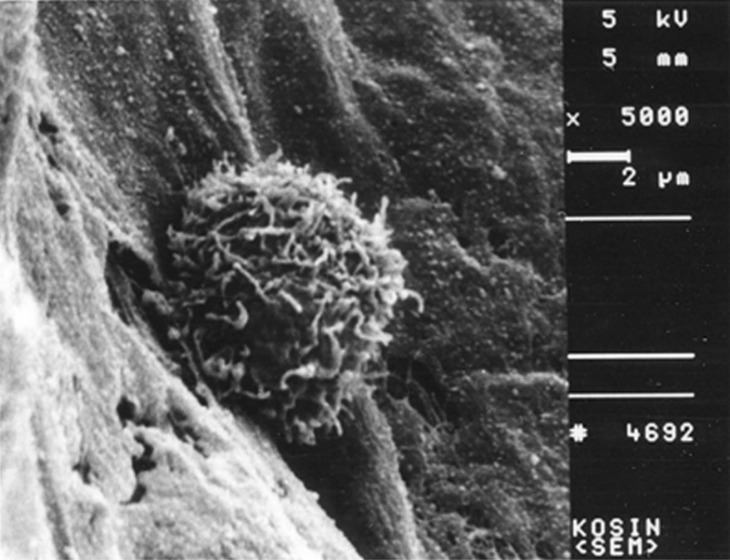
- Recent studies suggest that immunization with autologous dendritic cells (DCs) results in protective immunity and rejection of established tumors in various human malignancies. The purpose of this study is to determine whether DCs are generated from peripheral blood mononuclear cells (PBMNs) by using cytokines such as F1t-3 ligand (FL), granulocyte macrophage-colony stimulating factor (GM-CSF), IL-4, and TNF-alpha, and whether cytotoxic T cells activated against the thyroid cancer tissues by the DCs. Peripheral blood was obtained from 2 patients with thyroid cancer. DCs were established from PBMNs by culturing in the presence of FL, GM-CSF, IL-4, and TNF-alpha for 14 days. At day 14, the differentiated DCs was analyzed morphologically. The immunophenotypic features of DCs such as CDla, CD83, and CD86 were analyzed by immunofluorelescence microscopy. At day 18, DCs and T cells were incubated with thyroid cancer tissues or normal thyroid tissues for additional 4 days, respectively. DCs generated from the PBMNs showed the typical morphology of DCs. Activated cytotoxic T lymphocytes (CTLs) were observed also. DCs and the CTLs were attached to the cancer tissues on scanning electron microscope. The DCs activated the CTLs, which able to specifically attack the thyroid cancer. This study provides morphologic evidence that the coculture of T cells/cancer tissues activated the T cells and differentiated CTLs. The CTLs tightly adhered to cancer tissues and lysed cancer tissues vigorously. Therefore DCs could be used as potential vaccines in the immunotherapy.
Keyword
MeSH Terms
-
Coculture Techniques
Cytokines
Dendritic Cells
Electrons
Granulocyte-Macrophage Colony-Stimulating Factor
Granulocytes
Humans
Immunization
Immunotherapy
Interleukin-4
Microscopy
Rejection (Psychology)
Sensitivity and Specificity*
T-Lymphocytes
T-Lymphocytes, Cytotoxic
Thyroid Gland
Thyroid Neoplasms
Tumor Necrosis Factor-alpha
Vaccines
Cytokines
Granulocyte-Macrophage Colony-Stimulating Factor
Interleukin-4
Tumor Necrosis Factor-alpha
Vaccines
Figure
Reference
-
1. Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991; 9:271–296. PMID: 1910679.
Article2. Lee DH, Park JS, Eo WK, Kim WM, Kang K. Differentiation induction of dendritic cell phenotypes from human leukemic cell lines. Korean J Physiol Pharmacol. 2001; 5:79–86.3. McKenna HJ, de Vries P, Brasel K, Lyman SD, Williams DE. Effect of flt3 ligand on the ex vivo expansion of human CD34+ hematopoietic progenitor cells. Blood. 1995; 86:3413–3420. PMID: 7579445.
Article4. Young JC, Varma A, Digiusto D, Backer MP. Retention of quiescent hematopoietic cells with high proliferative potential during ex vivo stem cell culture. Blood. 1996; 87:545–556. PMID: 8555476.
Article5. Piacibello W, Sanavio F, Garetto L, Severino A, Bergandi D, Ferrario J, Fagioli F, Berger M, Aglietta M. Extensive amplification and self-renewal of human primitive hematopoietic stem cells from cord blood. Blood. 1997; 89:2644–2653. PMID: 9108381.
Article6. Lyman SD, James L, Johnson L, Brasel K, de Vries P, Escobar SS, Downey H, Splett RR, Beckmann MP, McKenna HJ. Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood. 1994; 83:2795–2801. PMID: 8180375.
Article7. Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998; 395:82–86. PMID: 9738502.
Article8. Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999; 285:221–227. PMID: 10398592.
Article9. Lanzavecchia A, Lezzi G, Viola A. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell. 1999; 96:1–4. PMID: 9989490.10. Lanzavecchia A, Sallusto F. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat Immunol. 2001; 2:487–492. PMID: 11376334.
Article12. Ockert D, Schmitz M, Hampl M, Rieber EP. Advances in cancer immunotherapy. Immunol Today. 1999; 20:63–65. PMID: 10098323.
Article13. Nestle FO, Burg G, Dummer R. New perspectives on immunobiology and immunotherapy of melanoma. Immunol Today. 1999; 20:5–7. PMID: 10081221.
Article14. Steinman RM. Dendritic cells and immune-based therapies. Exp Hematol. 1996; 24:859–862. PMID: 8690042.15. Schuler G, Steinman RM. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997; 186:1183–1187. PMID: 9379142.
Article16. Lotze MT. Getting to the source: dendritic cells as therapeutic reagents for the treatment of patients with cancer. Ann Surg. 1997; 226:1–5. PMID: 9242331.
Article17. Colaco CA. DC-based cancer immunotherapy: the sequel. Immunol Today. 1999; 20:197–198. PMID: 10203720.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interactions between Immune Cells and Tumor Cells
- Induction of Autologous Cancer-specific Cytotoxic T Lymphocytes using Dendritic Cells from Patients with Medullary Thyroid Carcinoma
- Circulating Lymphoma Cells in the Peripheral Blood from 4 Cases of Mantle and T Cell Types of Non-Hodgkin's Lymphoma: Light and Electron Microscopic Morphology
- Suppression of Pathogenic Autoreactive CD4+ T Cells by CD137-mediated Expansion of CD4+CD25+ Regulatory T Cells in Graves' Disease
- Tumor Cells and Cancer-Associated Fibroblasts: A Synergistic Crosstalk to Promote Thyroid Cancer