Korean J Urol.
2007 Jul;48(7):706-711. 10.4111/kju.2007.48.7.706.
The Expression of Proinflammatory Cytokine and Macrophage Migration Inhibitory Factor in a Rat Lipopolysaccharide-Induced Cystitis Model
- Affiliations
-
- 1Department of Urology, Soonchunhyang University College of Medicine, Bucheon, Korea. yhkuro@schbc.ac.kr
- KMID: 1990222
- DOI: http://doi.org/10.4111/kju.2007.48.7.706
Abstract
-
PURPOSE: We wanted to evaluate the changes of the expression of macrophage migration inhibitory factor(MIF) according to time, so we determined the semiquantitative score from immunostaining in a bladder inflammatory rat model.
MATERIALS AND METHODS
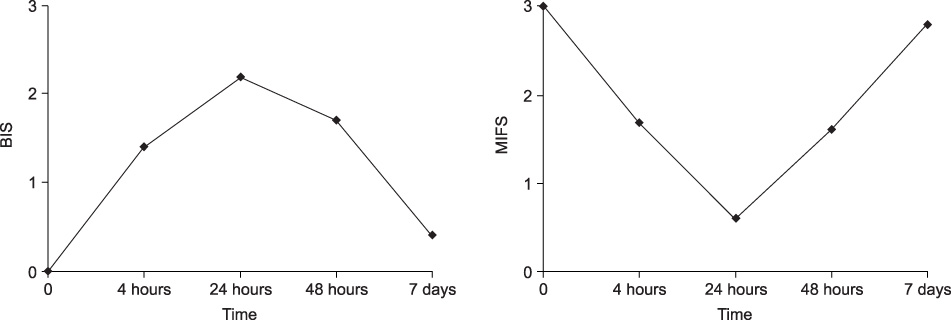
A total of 25 female Sprague-Dawley rats were divided into 5 groups according to the time course. Group 1 was the control group that was treated with an intravesical instillation of saline. Groups 2-5 were evaluated at 4 hours, 24 hours, 48 hours and 7 days after the instillation of lipopolysaccharide(LPS), respectively. H&E staining and immunohistochemical staining for MIF were performed after removing the bladder. A semiquantitative score was used to evaluate the cystitis(bladder inflammation score; BIS). The expression of MIF in the bladder was graded from 0 to 3+(MIF score; MIFS).
RESULTS
The staining of MIF was the most intense in the basal layer of the urothelium in the control group(BIS 0, MIFS 3). The degree of bladder inflammation was highest in group 3, and MIF was not expressed even in the urotherlium without inflammation(BIS 2.2, MIFS 0.6). The bladder inflammation was decreased after 48 hours, and the expression of MIF was increased after 48 hous(BIS 1.7, MIFS 1.6). The severity of bladder inflammation and the expression of MIS were significantly changed with the time course(p<0.001).
CONCLUSIONS
These results suggest that pre-formed MIF is stored in the cytoplasm of the basal cells in the urothelium, and it is released into the lumen of the bladder after a noxious stimulus like LPS.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee SJ, Lee SD, Cho IR, Sim BS, Lee JG, Kim CS, et al. Antimicrobial susceptibility of uropathogens causing acute uncomplicated cystitis in female outpatients in South Korea: a multicentre study in 2002. Int J Antimicrob Agents. 2004. 24:Suppl 1. S61–S64.2. Saban MR, Saban R, Hammond TG, Haak-Frendscho M, Steinberg H, Tengowski MW, et al. LPS-sensory peptide communication in experimental cystitis. Am J Physiol Renal Physiol. 2002. 282:202–210.3. Hamilton-Miller JM. Brufitt W, Hamilton-Miller JM, Bailey PR, editors. Pathogens other than Escherichia coli as aetiological agents in urinary tract infection. Urinary tract infections. 1998. 1st ed. London: Chapman and Hall Medical;59–74.4. Jung SY, Rhee HW, Lee DH, Bauer AJ. Effects of lipopolysaccharide on inflammation within the bladder muscle and muscle contractility in the rats. Korean J Urol. 1999. 40:1656–1662.5. Lee SJ, Lee CH, Kim SW, Cho YH, Yoon MS. Immunostimulating and prophylactic effect of Escherichia coli extract in a mouse model of lipopolysaccharide-induced cystitis. Korean J Urol. 2004. 45:1049–1055.6. Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003. 3:791–800.7. Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966. 153:80–82.8. David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966. 56:72–77.9. Weiser WY, Temple PA, Witek-Giannotti JS, Remold HG, Clark SC, David JR. Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc Natl Acad Sci U S A. 1989. 86:7522–7526.10. Bozza M, Satoskar AR, Lin G, Lu B, Humbles AA, Gerard C, David JR. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999. 189:341–346.11. Bozza M, Kolakowski LF Jr, Jenkins NA, Gilbert DJ, Copeland NG, David JR, et al. Structural characterization and chromosomal location of the mouse macrophage migration inhibitory factor gene and pseudogenes. Genomics. 1995. 27:412–419.12. Vera PL, Ordorica RC, Meyer-Siegler KL. Hydrochloric acid induced changes in macrophage migration inhibitory factor in the bladder, peripheral and central nervous system of the rat. J Urol. 2003. 170:623–627.13. Mitchell RA, Metz CN, Peng T, Bucala R. Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). Regulatory role in cell proliferation and glucocorticoid action. J Biol Chem. 1999. 274:18100–18106.14. Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001. 414:920–924.15. Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999. 190:1375–1382.16. Kleemann R, Hausser A, Geiger G, Mischke R, Burger-Kentischer A, Flieger O, et al. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature. 2000. 408:211–216.17. Meyer-Siegler KL, Vera PL. Substance P induced release of macrophage migration inhibitory factor from rat bladder epithelium. J Urol. 2004. 171:1698–1703.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Macrophage migration inhibitory factor: a potential therapeutic target for rheumatoid arthritis
- Wnt-C59 inhibits proinflammatory cytokine expression by reducing the interaction between β-catenin and NF-κB in LPS-stimulated epithelial and macrophage cells
- Association between Macrophage Migration Inhibitory Factor Gene Polymorphism and Rheumatoid Arthritis
- Activating transcription factor-3 induction is involved in the anti-inflammatory action of berberine in RAW264.7 murine macrophages
- Decreased Expression of Urethral Caveolin-1, -2, and -3 in the Rat Model of Overactive Bladder: Potential Mediator of Functional Interaction of Urethra and Urinary Bladder