Diabetes Metab J.
2011 Oct;35(5):489-496. 10.4093/dmj.2011.35.5.489.
Dietary Oleate Has Beneficial Effects on Every Step of Non-Alcoholic Fatty Liver Disease Progression in a Methionine- and Choline-Deficient Diet-Fed Animal Model
- Affiliations
-
- 1Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. bscha@yuhs.ac
- 2Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1857504
- DOI: http://doi.org/10.4093/dmj.2011.35.5.489
Abstract
- BACKGROUND
Non-alcoholic fatty liver disease (NAFLD) is increasingly recognized as a major cause of liver-related morbidity and mortality. The underlying mechanisms of disease progression remain poorly understood, and primary therapy of NAFLD is not yet established. We investigated the effects of dietary oleate on the development and progression of NAFLD in a methionine- and choline-deficient (MCD) diet-fed animal model.
METHODS
A total of 30 C57BL/6J mice were randomly divided into three groups (n=10 in each group) and fed various experimental diets for four weeks: chow, MCD diet, or OMCD (MCD diet with oleate, 0.5 mg/g/day). Liver samples were examined for steatohepatitis and fibrosis parameters and associated genes.
RESULTS
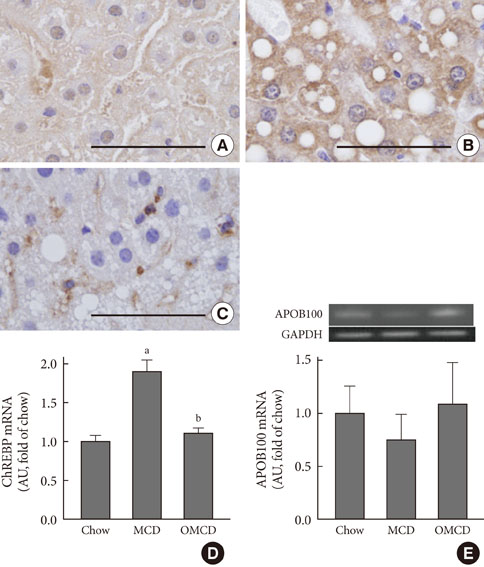
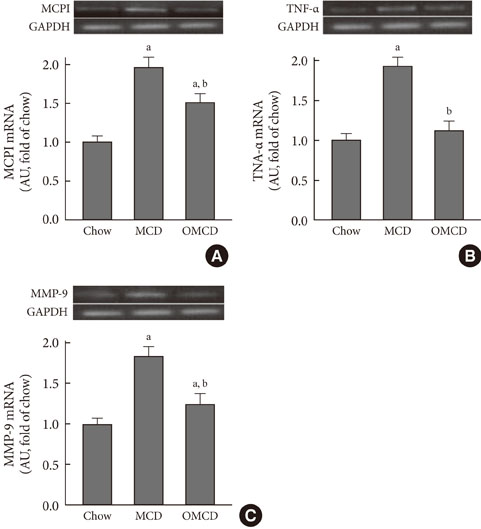
Additional dietary oleate dramatically reduced MCD diet-induced hepatic steatosis. Hepatic carbohydrate responsive element-binding protein was overexpressed in MCD diet-fed mice, and dietary oleate prevented this overexpression (P<0.001). Dietary oleate partially prevented MCD diet-induced serum level increases in aspartate aminotransferase and alanine aminotransferase (P<0.001, respectively). The mRNA expressions of hepatic monocyte chemoattractant protein 1, tumor necrosis factor-alpha and matrix metalloproteinase-9 were increased in MCD diet-fed mice, and this overexpression of inflammatory molecules was prevented by dietary oleate (P<0.001). Hepatic pericellular fibrosis was observed in MCD diet-fed mice, and dietary oleate prevented this fibrosis. Altogether, dietary oleate prevented MCD diet-induced hepatic steatosis, inflammation and fibrosis.
CONCLUSION
Dietary oleate has beneficial effects in every step of NAFLD development and progression and could be a nutritional option for NAFLD prevention and treatment.
MeSH Terms
-
Alanine Transaminase
Animals
Aspartate Aminotransferases
Chemokine CCL2
Corneal Dystrophies, Hereditary
Diet
Disease Progression
Fatty Acids, Monounsaturated
Fatty Liver
Fibrosis
Inflammation
Liver
Matrix Metalloproteinase 9
Mice
Models, Animal
Oleic Acid
RNA, Messenger
Tumor Necrosis Factor-alpha
Alanine Transaminase
Aspartate Aminotransferases
Chemokine CCL2
Corneal Dystrophies, Hereditary
Fatty Acids, Monounsaturated
Fatty Liver
Matrix Metalloproteinase 9
Oleic Acid
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006. 43:2 Suppl 1. S99–S112.2. Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010. 55:560–578.3. Cheung O, Sanyal AJ. Recent advances in nonalcoholic fatty liver disease. Curr Opin Gastroenterol. 2009. 25:230–237.4. Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008. 49:1068–1076.5. Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006. 43:163–172.6. Hussein O, Grosovski M, Lasri E, Svalb S, Ravid U, Assy N. Monounsaturated fat decreases hepatic lipid content in non-alcoholic fatty liver disease in rats. World J Gastroenterol. 2007. 13:361–368.7. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005. 115:1343–1351.8. Wong SH, Nestel PJ, Trimble RP, Storer GB, Illman RJ, Topping DL. The adaptive effects of dietary fish and safflower oil on lipid and lipoprotein metabolism in perfused rat liver. Biochim Biophys Acta. 1984. 792:103–109.9. Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009. 284:5637–5644.10. Abiru S, Migita K, Maeda Y, Daikoku M, Ito M, Ohata K, Nagaoka S, Matsumoto T, Takii Y, Kusumoto K, Nakamura M, Komori A, Yano K, Yatsuhashi H, Eguchi K, Ishibashi H. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver Int. 2006. 26:39–45.11. Oz HS, Im HJ, Chen TS, de Villiers WJ, McClain CJ. Glutathione-enhancing agents protect against steatohepatitis in a dietary model. J Biochem Mol Toxicol. 2006. 20:39–47.12. Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, Han JY, Kato S, Shimoda M, Oike Y, Tomizawa M, Makino S, Ohkura T, Saito H, Kumagai N, Nagata H, Ishii H, Hibi T. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006. 55:415–424.13. Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem. 1988. 263:2998–3004.14. Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006. 4:107–110.15. Stoeckman AK, Ma L, Towle HC. Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. J Biol Chem. 2004. 279:15662–15669.16. Rizki G, Arnaboldi L, Gabrielli B, Yan J, Lee GS, Ng RK, Turner SM, Badger TM, Pitas RE, Maher JJ. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res. 2006. 47:2280–2290.17. Larter CZ, Yeh MM, Haigh WG, Williams J, Brown S, Bell-Anderson KS, Lee SP, Farrell GC. Hepatic free fatty acids accumulate in experimental steatohepatitis: role of adaptive pathways. J Hepatol. 2008. 48:638–647.18. Ota T, Takamura T, Kurita S, Matsuzawa N, Kita Y, Uno M, Akahori H, Misu H, Sakurai M, Zen Y, Nakanuma Y, Kaneko S. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology. 2007. 132:282–293.19. Sahai A, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, Whitington PF. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004. 287:G1035–G1043.20. Dentin R, Benhamed F, Pegorier JP, Foufelle F, Viollet B, Vaulont S, Girard J, Postic C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J Clin Invest. 2005. 115:2843–2854.21. Wang H, Wollheim CB. ChREBP rather than USF2 regulates glucose stimulation of endogenous L-pyruvate kinase expression in insulin-secreting cells. J Biol Chem. 2002. 277:32746–32752.22. Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989. 169:1485–1490.23. Rollins BJ. Chemokines. Blood. 1997. 90:909–928.24. Jiménez-Gómez Y, Lopez-Miranda J, Blanco-Colio LM, Marin C, Perez-Martinez P, Ruano J, Paniagua JA, Rodriguez F, Egido J, Perez-Jimenez F. Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis. 2009. 204:e70–e76.25. Sun J, Feng A, Zhang Y, Sun S, Hu W, Yang M, Wei F, Qu X. Fucoidan increases TNF-alpha-induced MMP-9 secretion in monocytic cell line U937. Inflamm Res. 2010. 59:271–276.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Downregulation of GNAI3 Promotes the Pathogenesis of Methionine/Choline-Deficient Diet-Induced Nonalcoholic Fatty Liver Disease
- Comparative study of liver injury induced by high-fat methionine- and choline-deficient diet in ICR mice originating from three different sources
- Comparative study of fatty liver induced by methionine and choline-deficiency in C57BL/6N mice originating from three different sources
- Feasibility and Stability of Liver Biopsy before Treatment for Preclinical Nonalcoholic Fatty Liver Studies
- Effects of dietary taurine supplementation on plasma and liver lipids in OVX rats fed calcium-deficient diet