Korean J Physiol Pharmacol.
2015 May;19(3):291-297. 10.4196/kjpp.2015.19.3.291.
Bacterial PAMPs and Allergens Trigger Increase in [Ca2+]i-induced Cytokine Expression in Human PDL Fibroblasts
- Affiliations
-
- 1Department of Oral Biology, BK21 PLUS Project, Yonsei University College of Dentistry, Seoul 120-752, Korea. dmshin@yuhs.ac
- 2Department of Physiology, Gachon Graduate School of Medicine, Gachon University, Incheon 406-799, Korea.
- KMID: 1791432
- DOI: http://doi.org/10.4196/kjpp.2015.19.3.291
Abstract
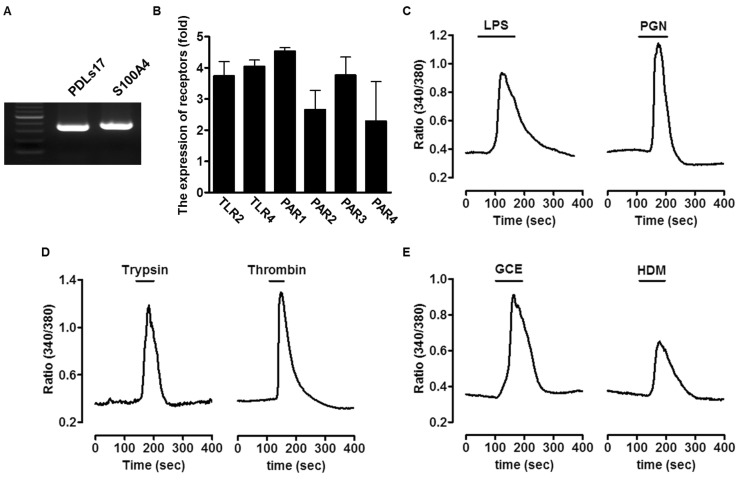
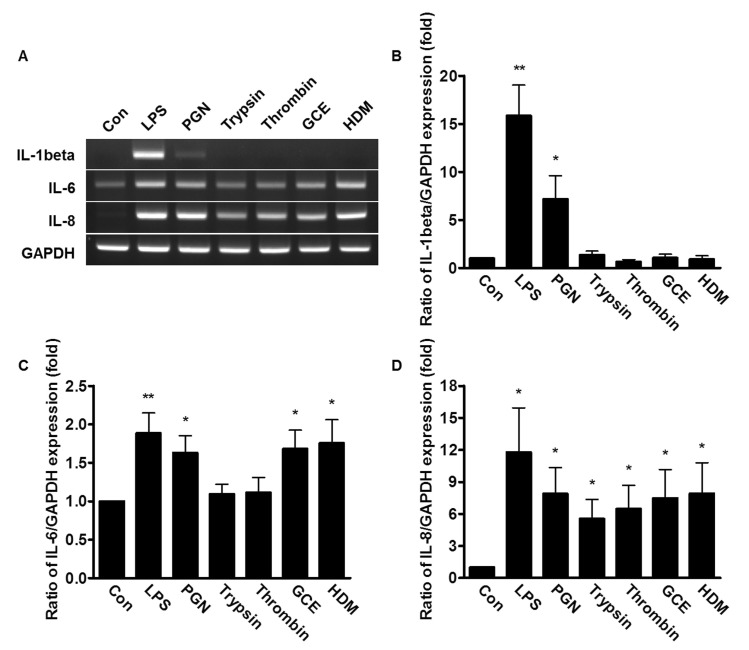
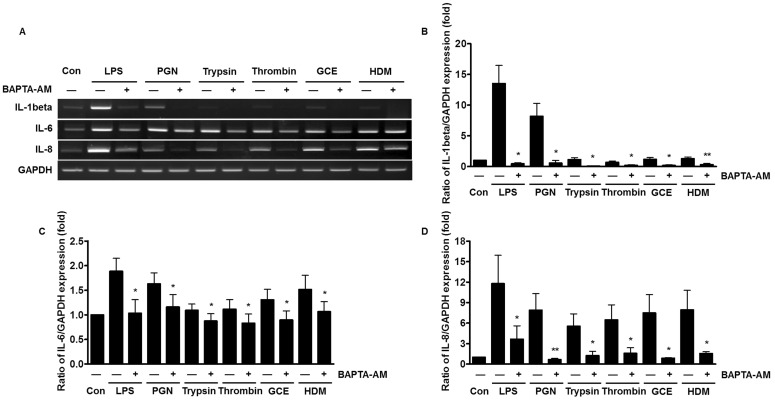
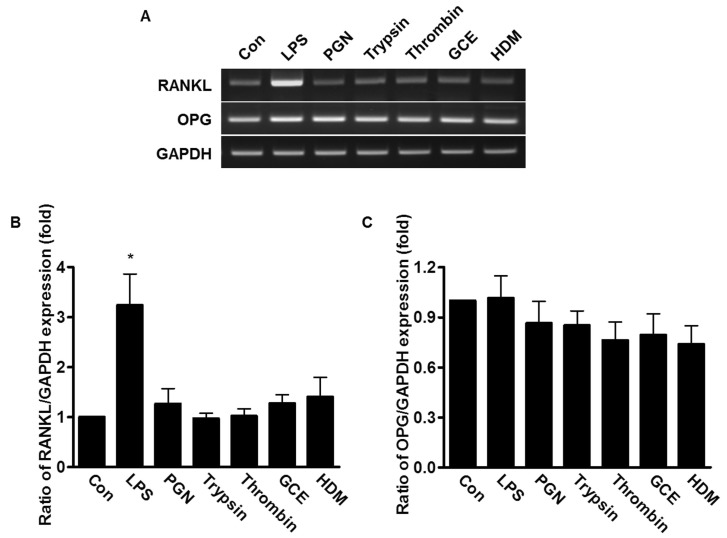
- An oral environment is constantly exposed to environmental factors and microorganisms. The periodontal ligament (PDL) fibroblasts within this environment are subject to bacterial infection and allergic reaction. However, how these condition affect PDL fibroblasts has yet to be elucidated. PDL fibroblasts were isolated from healthy donors. We examined using reverse transcription-polymerase chain reaction and measuring the intracellular Ca2+ concentration ([Ca2+]i). This study investigated the receptors activated by exogenous bacterial pathogens (Lipopolysaccharide and peptidoglycan) and allergens (German cockroach extract and house dust mite) as well as these pathogenic mediators-induced effects on the intracellular Ca2+ signaling in human PDL fibroblasts. Moreover, we evaluated the expression of pro-inflammatory cytokines (interleukin (IL)-1beta, IL-6, and IL-8) and bone remodeling mediators (receptor activator of NF-kappaB ligand and osteoprotegerin) and intracellular Ca2+-involved effect. Bacterial pathogens and allergic mediators induced increased expression of pro-inflammatory cytokines, and these results are dependent on intracellular Ca2+. However, bacterial pathogens and allergic mediators did not lead to increased expression of bone remodeling mediators, except lipopolysaccharide-induced effect on receptor activator of NF-kappaB ligand expression. These experiments provide evidence that a pathogens and allergens-induced increase in [Ca2+]i affects the inflammatory response in human PDL fibroblasts.
MeSH Terms
-
Allergens*
Bacterial Infections
Bone Remodeling
Calcium Signaling
Cockroaches
Cytokines
Dust
Fibroblasts*
Humans
Hypersensitivity
Inflammation
Interleukin-6
Interleukins
NF-kappa B
Periodontal Ligament
Receptor Activator of Nuclear Factor-kappa B
Tissue Donors
Allergens
Cytokines
Dust
Interleukin-6
Interleukins
NF-kappa B
Receptor Activator of Nuclear Factor-kappa B
Figure
Reference
-
1. Nogueira AV, Nokhbehsaim M, Eick S, Bourauel C, Jäger A, Jepsen S, Cirelli JA, Deschner J. Regulation of visfatin by microbial and biomechanical signals in PDL cells. Clin Oral Investig. 2014; 18:171–178.
Article2. Al-Ghutaimel H, Riba H, Al-Kahtani S, Al-Duhaimi S. Common periodontal diseases of children and adolescents. Int J Dent. 2014; 2014:850674. PMID: 25053946.
Article3. Jung UW, Kim CS, Choi SH, Kim S. Gingival coverage of iatrogenically denuded labial bone resulting from thermal trauma. Int J Periodontics Restorative Dent. 2013; 33:635–639. PMID: 23998159.
Article4. Kanjanamekanant K, Luckprom P, Pavasant P. Mechanical stress-induced interleukin-1beta expression through adenosine triphosphate/P2X7 receptor activation in human periodontal ligament cells. J Periodontal Res. 2013; 48:169–176. PMID: 22881405.
Article5. Nakao A, Kajiya H, Fukushima H, Fukushima A, Anan H, Ozeki S, Okabe K. PTHrP induces Notch signaling in periodontal ligament cells. J Dent Res. 2009; 88:551–556. PMID: 19587161.
Article6. Gher ME. Changing concepts. The effects of occlusion on periodontitis. Dent Clin North Am. 1998; 42:285–299. PMID: 9597338.7. Jönsson D, Nebel D, Bratthall G, Nilsson BO. The human periodontal ligament cell: a fibroblast-like cell acting as an immune cell. J Periodontal Res. 2011; 46:153–157. PMID: 21118418.
Article8. Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006; 86:9–22. PMID: 16357866.
Article9. Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004; 4:499–511. PMID: 15229469.
Article10. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006; 124:783–801. PMID: 16497588.
Article11. Hong JH, Hong JY, Park B, Lee SI, Seo JT, Kim KE, Sohn MH, Shin DM. Chitinase activates protease-activated receptor-2 in human airway epithelial cells. Am J Respir Cell Mol Biol. 2008; 39:530–535. PMID: 18474671.
Article12. Cho HJ, Choi JY, Yang YM, Hong JH, Kim CH, Gee HY, Lee HJ, Shin DM, Yoon JH. House dust mite extract activates apical Cl(-) channels through protease-activated receptor 2 in human airway epithelia. J Cell Biochem. 2010; 109:1254–1263. PMID: 20186875.13. Hong JH, Lee SI, Kim KE, Yong TS, Seo JT, Sohn MH, Shin DM. German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. J Allergy Clin Immunol. 2004; 113:315–319. PMID: 14767448.
Article14. Jeong SK, Kim HJ, Youm JK, Ahn SK, Choi EH, Sohn MH, Kim KE, Hong JH, Shin DM, Lee SH. Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. J Invest Dermatol. 2008; 128:1930–1939. PMID: 18305573.
Article15. Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol. 2001; 167:1014–1021. PMID: 11441110.
Article16. Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993; 61:4569–4574. PMID: 8406853.
Article17. Kauffman HF, Tomee JF, van de Riet MA, Timmerman AJ, Borger P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol. 2000; 105:1185–1193. PMID: 10856154.
Article18. Liu Y, Wu Z, Zhang X, Ni J, Yu W, Zhou Y, Nakanishi H. Leptomeningeal cells transduce peripheral macrophages inflammatory signal to microglia in reponse to Porphyromonas gingivalis LPS. Mediators Inflamm. 2013; 2013:407562. PMID: 24363500.19. Sloan AJ, Taylor SY, Smith EL, Roberts JL, Chen L, Wei XQ, Waddington RJ. A novel ex vivo culture model for inflammatory bone destruction. J Dent Res. 2013; 92:728–734. PMID: 23857868.
Article20. Aki D, Minoda Y, Yoshida H, Watanabe S, Yoshida R, Takaesu G, Chinen T, Inaba T, Hikida M, Kurosaki T, Saeki K, Yoshimura A. Peptidoglycan and lipopolysaccharide activate PLCgamma2, leading to enhanced cytokine production in macrophages and dendritic cells. Genes Cells. 2008; 13:199–208. PMID: 18233961.21. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003; 4:517–529. PMID: 12838335.
Article22. Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012; 4.
Article23. Son A, Shin DM, Hong JH. Peptidoglycan induces the production of interleukin-8 via calcium signaling in human gingival epithelium. Korean J Physiol Pharmacol. 2015; 19:51–57. PMID: 25605997.
Article24. Richards D, Rutherford RB. The effects of interleukin 1 on collagenolytic activity and prostaglandin-E secretion by human periodontal-ligament and gingival fibroblast. Arch Oral Biol. 1988; 33:237–243. PMID: 3261162.
Article25. Chamizo C, Moreno J, Alvar J. Semi-quantitative analysis of cytokine expression in asymptomatic canine leishmaniasis. Vet Immunol Immunopathol. 2005; 103:67–75. PMID: 15626462.
Article26. Marone M, Mozzetti S, De Ritis D, Pierelli L, Scambia G. Semiquantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biol Proced Online. 2001; 3:19–25. PMID: 12734582.
Article27. Hajishengallis G, Sharma A, Russell MW, Genco RJ. Interactions of oral pathogens with toll-like receptors: possible role in atherosclerosis. Ann Periodontol. 2002; 7:72–78. PMID: 16013219.
Article28. Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, Stewart GA. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 2002; 169:4572–4578. PMID: 12370395.
Article29. Bhat RK, Page K, Tan A, Hershenson MB. German cockroach extract increases bronchial epithelial cell interleukin-8 expression. Clin Exp Allergy. 2003; 33:35–42. PMID: 12534547.
Article30. Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev. 2012; 92:39–74. PMID: 22298651.31. Son A, Kim MS, Jo H, Byun HM, Shin DM. Effects of inositol 1,4,5-triphosphate on osteoclast differentiation in RANKL-induced osteoclastogenesis. Korean J Physiol Pharmacol. 2012; 16:31–36. PMID: 22416217.
Article32. Nibali L, Fedele S, D'Aiuto F, Donos N. Interleukin-6 in oral diseases: a review. Oral Dis. 2012; 18:236–243. PMID: 22050374.
Article33. Kaur S, White S, Bartold PM. Periodontal disease and rheumatoid arthritis: a systematic review. J Dent Res. 2013; 92:399–408. PMID: 23525531.34. Costa PP, Trevisan GL, Macedo GO, Palioto DB, Souza SL, Grisi MF, Novaes AB Jr, Taba M Jr. Salivary interleukin-6, matrix metalloproteinase-8, and osteoprotegerin in patients with periodontitis and diabetes. J Periodontol. 2010; 81:384–391. PMID: 20192865.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- House Dust Mite Extract Induces PLC/IP3-dependent Ca2+ Signaling and IL-8 Expression in Human Gingival Epithelial Cells
- Dexmedetomidine Modulates Histamine-induced Ca2+ Signaling and Pro-inflammatory Cytokine Expression
- Biochemical characteristics of human periodontal ligament cells in vitro
- Candida spp.- Induced Cytokine Gene Expression on Mouse Peritoneal Macrophages and NIH 3T3 Fibroblasts
- The effect of mineral trioxide aggregate on the production of growth factors and cytokine by human periodontal ligament fibroblasts