The effect of mineral trioxide aggregate on the production of growth factors and cytokine by human periodontal ligament fibroblasts
- Affiliations
-
- 1Department of Conservative Dentistry, College of Dentistry, Seoul National University, Korea. jimin525@snu.ac.kr
- KMID: 2175868
- DOI: http://doi.org/10.5395/JKACD.2007.32.3.191
Abstract
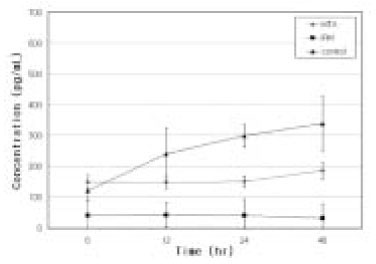
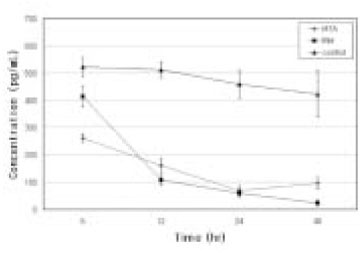
- Mineral trioxide aggregate (MTA) would influence healing of periapical tissues by modulating the production of growth factors and cytokines from PDL fibroblasts, however, the studies are insufficient. Therefore, the purpose of this study was to monitor the expression of transforming growth factor-beta1 (TGF- beta1), fibroblast growth factor-2 (FGF-2), and interleukin-6 (IL-6) from PDL fibroblasts in the presence of MTA. The human PDL fibroblasts were seeded onto the set MTA or IRM at a level of 1 x 10(5) cells per unit well, and further incubated for 6, 12, 24, and 48 hours. The levels of TGF-beta1, FGF-2, and IL-6 from the supernatant were measured by enzyme-linked immunosorbent assay (ELISA). The data were analyzed using one-way ANOVA. The level of TGF-beta1 was down-regulated when the cells were grown in the presence of MTA except at 6 hours. The levels of FGF-2 release were significantly suppressed when PDL fibroblasts were grown in the presence of MTA or IRM at all time intervals (p < 0.05). The expressions of IL-6 from MTA treated cells were comparable to those of untreated control cells throughout the observation periods. We presume that this material inhibits the stimulatory function of growth factors on granulation tissue formation and in turn, it promotes the healing process modulated by other bone-remodeling cells.
Keyword
MeSH Terms
-
Cytokines
Enzyme-Linked Immunosorbent Assay
Fibroblast Growth Factor 2
Fibroblasts*
Granulation Tissue
Humans*
Intercellular Signaling Peptides and Proteins*
Interleukin-6
Periapical Tissue
Periodontal Ligament*
Transforming Growth Factor beta1
Pemetrexed
Cytokines
Fibroblast Growth Factor 2
Intercellular Signaling Peptides and Proteins
Interleukin-6
Transforming Growth Factor beta1
Figure
Cited by 4 articles
-
Effects of condensation techniques and canal sizes on the microleakage of orthograde MTA apical plug in simulated canals
Deuk-Lim Nam, Jeong-Kil Park, Bock Hur, Hyeon-Cheol Kim
J Korean Acad Conserv Dent. 2009;34(3):208-214. doi: 10.5395/JKACD.2009.34.3.208.The effect of several root-end filling materials on MG63 osteoblast-like cells
Jeong-Ho Lee, Won-Jun Shon, WooCheol Lee, Seung-Ho Baek
J Korean Acad Conserv Dent. 2010;35(3):222-228. doi: 10.5395/JKACD.2010.35.3.222.Biocompatibility of experimental mixture of mineral trioxide aggregate and glass ionomer cement
Min-Jae Oh, Yu-Na Jeong, In-Ho Bae, So-Young Yang, Bum-Jun Park, Jeong-Tae Koh, Yun-Chan Hwang, In-Nam Hwang, Won-Mann Oh
J Korean Acad Conserv Dent. 2010;35(5):359-367. doi: 10.5395/JKACD.2010.35.5.359.Biocompatibility of bioaggregate cement on human pulp and periodontal ligament (PDL) derived cells
Choo-Ryung Chung, Euiseong Kim, Su-Jung Shin
J Korean Acad Conserv Dent. 2010;35(6):473-478. doi: 10.5395/JKACD.2010.35.6.473.
Reference
-
1. Gutmann JL, Harrison JW. Surgical endodontics. 1994. St. Louis, MO: IEA Inc..2. Dorn SO, Gartner AH. Retrograde filling materials : a retrospective success-failure study of amalgam, EBA, and IRM. J Endod. 1990. 16:391–393.
Article3. Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993. 19:591–595.
Article4. Tang HM, Torabinejad M, Kettering JD. Leakage evaluation of root end filling materials using endotoxin. J Endod. 2002. 28:5–7.
Article5. Torabinejad M, Pitt Ford TR, McKendry DJ, Abedi HR, Miller DA, Kariyawasam SP. Histologic assessment of mineral trioxide aggregate as a root-end filling in monkeys. J Endod. 1997. 23:225–228.
Article6. Torabinejad M, Ford TR, Abedi HR, Kariyawasam SP, Tang HM. Tissue reaction to implanted root-end filling materials in the tibia and mandible of guinea pigs. J Endod. 1998. 24:468–471.
Article7. Economides N, Pantelidou O, Kokkas A, Tziafas D. Short-term periradicular tissue response to mineral trioxide aggregate (MTA) as root-end filling material. Int Endod J. 2003. 36:44–48.
Article8. Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to Mineral Trioxide Aggregate. J Endod. 1998. 24:543–547.
Article9. Mitchell PJ, Pitt Ford TR, Torabinejad M, McDonald F. Osteoblast biocompatibility of mineral trioxide aggregate. Biomaterials. 1999. 20:167–173.
Article10. Balto HA. Attachment and morphological behavior of human periodontal ligament fibroblasts to mineral trioxide aggregate: a scanning electron microscope study. J Endod. 2004. 30:25–29.
Article11. Thomson TS, Berry JE, Somerman MJ, Kirkwood KL. Cementoblasts maintain expression of osteocalcin in the presence of mineral trioxide aggregate. J Endod. 2003. 29:407–412.
Article12. . The potential role of growth and differentiation factors in periodontal regeneration. J Periodontol. 1996. 67:545–553.13. Oringer RJ. Biological mediators for periodontal and bone regeneration. Compend Contin Educ Dent. 2002. 501–504. 506–510. 512 passimquiz 518.14. Centrella M, McCarthy TL, Canalis E. Transforming growth factor beta is a bifunctional regulator of replication and collagen synthesis in osteoblast-enriched cell cultures from fetal rat bone. J Biol Chem. 1987. 262:2869–2874.
Article15. Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998. 9:248–266.
Article16. Lyngstadaas SP, Lundberg E, Ekdahl H, Andersson C, Gestrelius S. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J Clin Periodontol. 2001. 28:181–188.
Article17. Koh ET, Torabinejad M, Pitt Ford TR, Brady K, McDonald F. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. J Biomed Mater Res. 1997. 37:432–439.
Article18. Boyko GA, Melcher AH, Brunette DM. Formation of new periodontal ligament by periodontal ligament cells implanted in vivo after culture in vitro. A preliminary study of transplanted roots in the dog. J Periodontal Res. 1981. 16:73–88.
Article19. Melcher AH. On the repair potential of periodontal tissue. J Periodontol. 1976. 47:256–260.20. McCulloch CAG, Bordin S. Role of fibroblast subpopulations in periodontal physiology and pathology. J Periodont Res. 1991. 26:144–154.
Article21. Corcoran JF, Sieraski SM, Ellison RL. Osseous healing kinetics after picoectomy in monkeys. II. A quantitative histologic appraisal. J Endod. 1985. 11:269–274.
Article22. Harrison JW, Jurosky KA. Wound healing in the tissues of the periodontium following periradicular surgery. III. The osseous excisional wound. J Endod. 1992. 18:76–81.
Article23. Regan JD, Gutmann JL, Witherspoon DE. Comparison of Diaket and MTA when used as root-end filling materials to support regeneration of the periradicular tissues. Int Endod J. 2002. 35:840–847.
Article24. Keiser K, Johnson CC, Tipton DA. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblast. J Endod. 2000. 26:288–291.
Article25. Graves DT, Cochran DL. Periodontal regeneration with polypeptide growth factors. Curr Opin Periodontol. 1994. 1:178–186.26. Kissin EY, Lemaire R, Korn JH, Lafyatis R. Transforming growth factor beta induces fibroblast fibrillin-1 matrix formation. Arthritis Rheum. 2002. 46:3000–3009.
Article27. Piche JE, Graves DT. Study of the growth factor requirements of human bone-derived cells: a comparison with human fibroblasts. Bone. 1989. 10:131–138.
Article28. Chenu C, Pfeilschifter J, Mundy GR, Roodman GD. Transforming growth factor beta inhibits formation of osteoclast-like cells in long-term human marrow cultures. Proc Natl Acad Sci. 1988. 85:5683–5687.
Article29. Okuda K, Kawase T, Momose M, Murata M, Saito Y, Suzuki H, Wolff LF, Yoshie H. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol. 2003. 74:849–857.
Article30. Takayama S, Murakami S, Miki Y, Ikezawa K, Tasaka S, Terashima A, Asano T, Okada H. Effects of basic fibroblast growth factor on human periodontal ligament cells. J Periodontal Res. 1997. 32:667–675.
Article31. Ishimi Y, Miyaura C, Jin CH, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990. 145:3297–3303.32. Haglund R, He J, Jarvis J, Safavi KE, Spangberg LSW, Zhu Q. Effects of root-end filling materials on fibroblasts and macrophages in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003. 95:739–745.
Article33. Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to mineral trioxide aggregate. J Endod. 1998. 24:543–547.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- White mineral trioxide aggregate mixed with calcium chloride dihydrate: chemical analysis and biological properties
- Effect of high glucose on the prostaglandin E2 production in human gingival fibroblasts and periodontal ligament cells
- Effects of Direct Cell Contact Between Monocytes and Fibroblasts on the Interleukin-6 Production and Cell Proliferation of Human Gingival and Periodontal Ligament Fibroblasts
- Effect of Inorganic Polyphosphate on Cultured Periodontal Ligament Cells
- Effect of various cytokines on the production of prostaglandin E2 leukotriene B4 and collagenase in human periodontal ligament fibroblasts in vitro