Blood Res.
2015 Mar;50(1):33-39. 10.5045/br.2015.50.1.33.
Anti-leukemic properties of deferasirox via apoptosis in murine leukemia cell lines
- Affiliations
-
- 1Department of Pediatrics, Catholic Blood and Marrow Transplantation Center, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. cngped@catholic.ac.kr
- KMID: 1787910
- DOI: http://doi.org/10.5045/br.2015.50.1.33
Abstract
- BACKGROUND
Although deferasirox (DFX) is reported to have anti-tumor effects, its anti-leukemic activity remains unclear. We evaluated the effect of DFX treatment on two murine lymphoid leukemia cell lines, and clarified the mechanisms underlying its potential anti-leukemic activity.
METHODS
L1210 and A20 murine lymphoid leukemia cell lines were treated with DFX. Cell viability and apoptosis were evaluated by the 3-(4,5-dimethylthaizol-2-yl)-5-(3-carboxymethylphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay and fluorescence-activated cell sorting (FACS) analysis, respectively. Immunoblotting was performed to detect the expression of key apoptotic proteins.
RESULTS
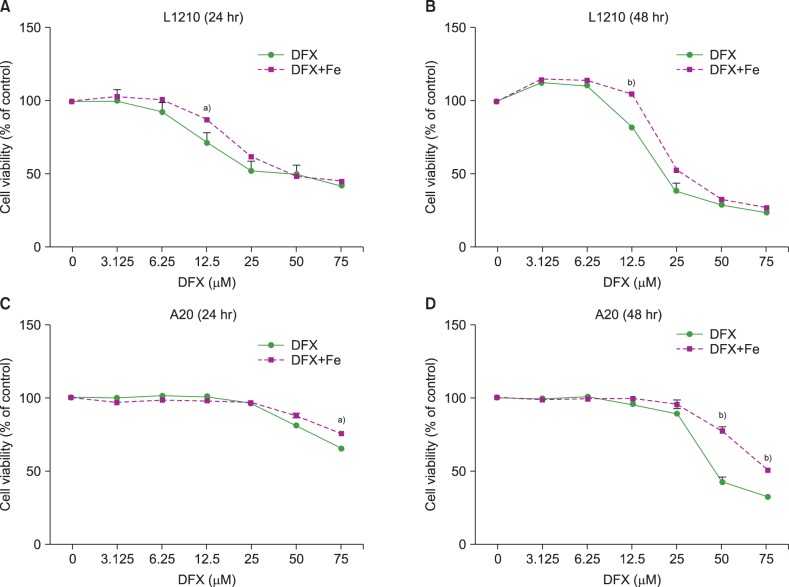
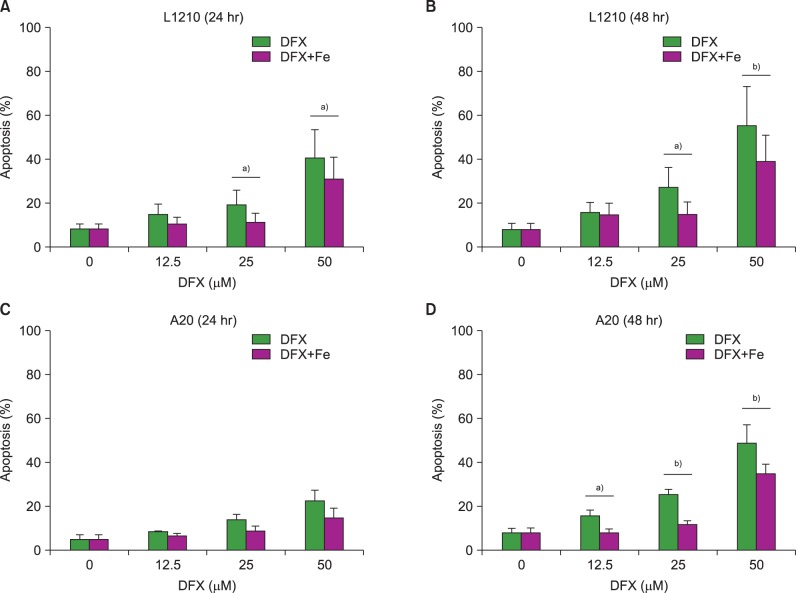
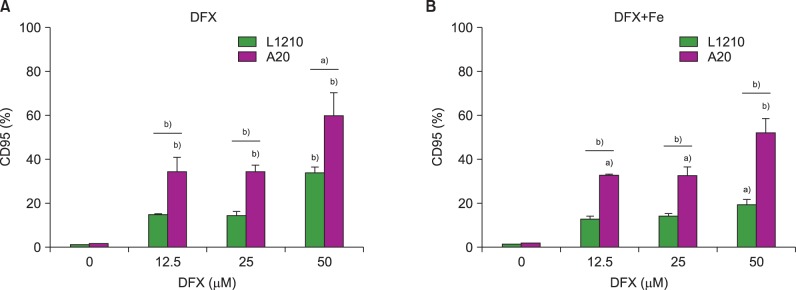
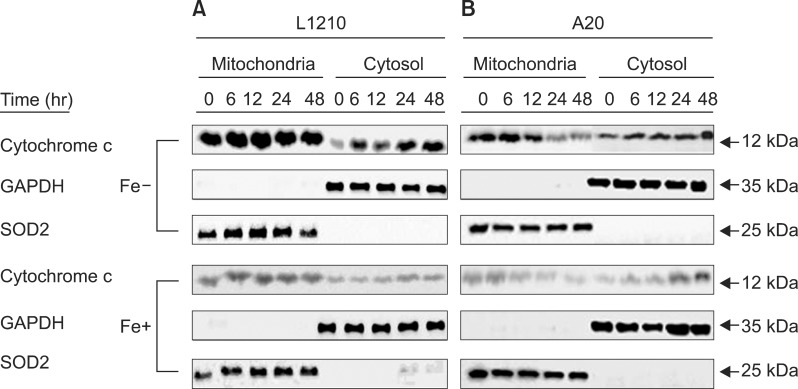
In dose- and time-dependent manner, DFX decreased viability and increased apoptosis of murine leukemic cells. Fas expression was significantly higher in A20 cells than in L1210 cells at all DFX concentrations tested. Although both cell lines exhibited high caspase 3 and caspase 9 expression, a critical component of the intrinsic mitochondrial apoptotic pathway, expression was greater in L1210 cells. In contrast, caspase 8, a key factor in the extrinsic apoptotic pathway, showed greater expression in A20 cells. Cytochrome c expression was significantly higher in L1210 cells. In both cell lines, co-treatment with ferric chloride and DFX diminished the expression of these intracellular proteins, as compared to DFX treatment alone.
CONCLUSION
Treatment with DFX increased caspase-dependent apoptosis in two murine lymphoid leukemia cell lines, with differing apoptotic mechanisms in each cell line.
Keyword
MeSH Terms
Figure
Reference
-
1. Ali S, Pimentel JD, Munoz J, et al. Iron overload in allogeneic hematopoietic stem cell transplant recipients. Arch Pathol Lab Med. 2012; 136:532–538. PMID: 22540302.
Article2. Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001; 22:2171–2179. PMID: 11913479.3. Lee DH, Jang PS, Chung NG, Cho B, Jeong DC, Kim HK. Deferasirox shows in vitro and in vivo antileukemic effects on murine leukemic cell lines regardless of iron status. Exp Hematol. 2013; 41:539–546. PMID: 23415674.4. Cunningham-Rundles S, Giardina PJ, Grady RW, Califano C, McKenzie P, De Sousa M. Effect of transfusional iron overload on immune response. J Infect Dis. 2000; 182(Suppl 1):S115–S121. PMID: 10944493.
Article5. Malcovati L. Red blood cell transfusion therapy and iron chelation in patients with myelodysplastic syndromes. Clin Lymphoma Myeloma. 2009; 9(Suppl 3):S305–S311. PMID: 19778858.6. Smith-Whitley K, Thompson AA. Indications and complications of transfusions in sickle cell disease. Pediatr Blood Cancer. 2012; 59:358–364. PMID: 22566388.
Article7. Neufeld EJ. Oral chelators deferasirox and deferiprone for transfusional iron overload in thalassemia major: new data, new questions. Blood. 2006; 107:3436–3441. PMID: 16627763.
Article8. Balooch FD, Fatemi SJ, Iranmanesh M, Hosseinkhani B. Combined chelation therapy with deferasirox and desferrioxamine in removing lead from rats. Am J Pharmacol Toxicol. 2013; 8:134–140.
Article9. Peccatori J, Ciceri F. Allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2010; 95:857–859. PMID: 20513804.
Article10. Dayani PN, Bishop MC, Black K, Zeltzer PM. Desferoxamine (DFO)-mediated iron chelation: rationale for a novel approach to therapy for brain cancer. J Neurooncol. 2004; 67:367–377. PMID: 15164994.11. Marx JJ. Iron and infection: competition between host and microbes for a precious element. Best Pract Res Clin Haematol. 2002; 15:411–426. PMID: 12401315.
Article12. Messa E, Carturan S, Maffè C, et al. Deferasirox is a powerful NF-kappaB inhibitor in myelodysplastic cells and in leukemia cell lines acting independently from cell iron deprivation by chelation and reactive oxygen species scavenging. Haematologica. 2010; 95:1308–1316. PMID: 20534700.13. Ohyashiki JH, Kobayashi C, Hamamura R, Okabe S, Tauchi T, Ohyashiki K. The oral iron chelator deferasirox represses signaling through the mTOR in myeloid leukemia cells by enhancing expression of REDD1. Cancer Sci. 2009; 100:970–977. PMID: 19298223.
Article14. Kushner JP, Porter JP, Olivieri NF. Secondary iron overload. Hematology Am Soc Hematol Educ Program. 2001; 47–61. PMID: 11722978.
Article15. Goldberg SL. Novel treatment options for transfusional iron overload in patients with myelodysplastic syndromes. Leuk Res. 2007; 31(Suppl 3):S16–S22. PMID: 18037414.
Article16. Bedford MR, Ford SJ, Horniblow RD, Iqbal TH, Tselepis C. Iron chelation in the treatment of cancer: a new role for deferasirox? J Clin Pharmacol. 2013; 53:885–891. PMID: 23740857.
Article17. Fukushima T, Kawabata H, Nakamura T, et al. Iron chelation therapy with deferasirox induced complete remission in a patient with chemotherapy-resistant acute monocytic leukemia. Anticancer Res. 2011; 31:1741–1744. PMID: 21617233.18. Becton DL, Roberts B. Antileukemic effects of deferoxamine on human myeloid leukemia cell lines. Cancer Res. 1989; 49:4809–4812. PMID: 2758414.19. Kim JL, Kang HN, Kang MH, Yoo YA, Kim JS, Choi CW. The oral iron chelator deferasirox induces apoptosis in myeloid leukemia cells by targeting caspase. Acta Haematol. 2011; 126:241–245. PMID: 21951998.
Article20. Estrov Z, Tawa A, Wang XH, et al. In vitro and in vivo effects of deferoxamine in neonatal acute leukemia. Blood. 1987; 69:757–761. PMID: 3493042.
Article21. Ford SJ, Obeidy P, Lovejoy DB, et al. Deferasirox (ICL670A) effectively inhibits oesophageal cancer growth in vitro and in vivo. Br J Pharmacol. 2013; 168:1316–1328. PMID: 23126308.22. Gaboriau F, Leray AM, Ropert M, et al. Effects of deferasirox and deferiprone on cellular iron load in the human hepatoma cell line HepaRG. Biometals. 2010; 23:231–245. PMID: 19997770.
Article23. Gharagozloo M, Khoshdel Z, Amirghofran Z. The effect of an iron (III) chelator, silybin, on the proliferation and cell cycle of Jurkat cells: a comparison with desferrioxamine. Eur J Pharmacol. 2008; 589:1–7. PMID: 18619590.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of oral iron chelator deferasirox on human malignant lymphoma cells
- Use of deferasirox, an iron chelator, to overcome imatinib resistance of chronic myeloid leukemia cells
- Apoptosis Induction by Curcumin in Human Myelogenous Leukemia Cell Lines
- Effect of G-CSF on Myeloid Leukemic Cell Lines after Treatment with Ara-C
- Induction of hypoxia-inducible factor-1alpha inhibits drug-induced apoptosis in the human leukemic cell line HL-60