Korean J Hematol.
2012 Sep;47(3):194-201. 10.5045/kjh.2012.47.3.194.
Effects of oral iron chelator deferasirox on human malignant lymphoma cells
- Affiliations
-
- 1Department of Internal Medicine, Korea University Guro Hospital, Seoul, Korea. bonnie@korea.ac.kr
- 2Graduate School of Medicine, Korea University College of Medicine, Seoul, Korea.
- KMID: 2251953
- DOI: http://doi.org/10.5045/kjh.2012.47.3.194
Abstract
- BACKGROUND
Iron is essential for cell proliferation and viability. It has been reported that iron depletion by a chelator inhibits proliferation of some cancer cells. Deferasirox is a new oral iron chelator, and a few reports have described its effects on lymphoma cells. The goal of this study was to determine the anticancer effects of deferasirox in malignant lymphoma cell lines.
METHODS
Three human malignant lymphoma cell lines (NCI H28:N78, Ramos, and Jiyoye) were treated with deferasirox at final concentrations of 20, 50, or 100 microM. Cell proliferation was evaluated by an MTT assay, and cell cycle and apoptosis were analyzed by flow cytometry. Western blot analysis was performed to determine the relative activity of various apoptotic pathways. The role of caspase in deferasirox-induced apoptosis was investigated using a luminescent assay.
RESULTS
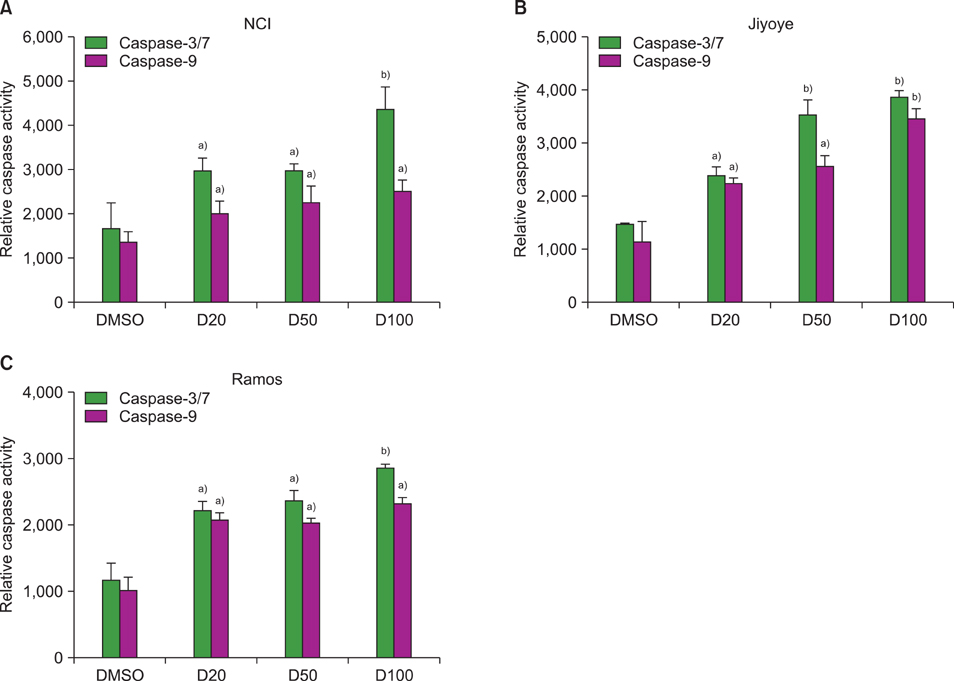
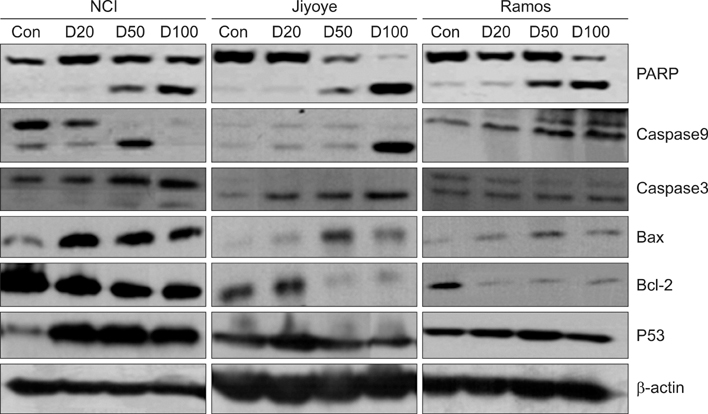
The MTT assay showed that deferasirox had dose-dependent cytotoxic effects on all 3 cell lines. Cell cycle analysis showed that the sub-G1 portion increased in all 3 cell lines as the concentration of deferasirox increased. Early apoptosis was also confirmed in the treated cells by Annexin V and PI staining. Western blotting showed an increase in the cleavage of PARP, caspase 3/7, and caspase 9 in deferasirox-treated groups.
CONCLUSION
We demonstrated that deferasirox, a new oral iron-chelating agent, induced early apoptosis in human malignant lymphoma cells, and this apoptotic effect is dependent on the caspase-3/caspase-9 pathway.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Anti-leukemic properties of deferasirox via apoptosis in murine leukemia cell lines
Sol-Rim Jeon, Jae-Wook Lee, Pil-Sang Jang, Nack-Gyun Chung, Bin Cho, Dae-Chul Jeong
Blood Res. 2015;50(1):33-39. doi: 10.5045/br.2015.50.1.33.
Reference
-
1. Zhang AS, Enns CA. Iron homeostasis: recently identified proteins provide insight into novel control mechanisms. J Biol Chem. 2009. 284:711–715.
Article2. Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008. 112:219–230.
Article3. De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol. 2008. 9:72–81.
Article4. Yu Y, Wong J, Lovejoy DB, Kalinowski DS, Richardson DR. Chelators at the cancer coalface: desferrioxamine to Triapine and beyond. Clin Cancer Res. 2006. 12:6876–6883.
Article5. Kalinowski DS, Yu Y, Sharpe PC, et al. Design, synthesis, and characterization of novel iron chelators: structure-activity relationships of the 2-benzoylpyridine thiosemicarbazone series and their 3-nitrobenzoyl analogues as potent antitumor agents. J Med Chem. 2007. 50:3716–3729.
Article6. Triantafyllou A, Liakos P, Tsakalof A, et al. The flavonoid quercetin induces hypoxia-inducible factor-1alpha (HIF-1alpha) and inhibits cell proliferation by depleting intracellular iron. Free Radic Res. 2007. 41:342–356.
Article7. Gharagozloo M, Khoshdel Z, Amirghofran Z. The effect of an iron (III) chelator, silybin, on the proliferation and cell cycle of Jurkat cells: a comparison with desferrioxamine. Eur J Pharmacol. 2008. 589:1–7.
Article8. Chaston TB, Lovejoy DB, Watts RN, Richardson DR. Examination of the antiproliferative activity of iron chelators: multiple cellular targets and the different mechanism of action of triapine compared with desferrioxamine and the potent pyridoxal isonicotinoyl hydrazone analogue 311. Clin Cancer Res. 2003. 9:402–414.9. Choudhari SR, Khan MA, Harris G, et al. Deactivation of Akt and STAT3 signaling promotes apoptosis, inhibits proliferation, and enhances the sensitivity of hepatocellular carcinoma cells to an anticancer agent, Atiprimod. Mol Cancer Ther. 2007. 6:112–121.
Article10. Valle P, Timeus F, Piglione M, et al. Effect of different exposures to desferrioxamine on neuroblastoma cell lines. Pediatr Hematol Oncol. 1995. 12:439–446.
Article11. Brard L, Granai CO, Swamy N. Iron chelators deferoxamine and diethylenetriamine pentaacetic acid induce apoptosis in ovarian carcinoma. Gynecol Oncol. 2006. 100:116–127.
Article12. Messa E, Carturan S, Maffè C, et al. Deferasirox is a powerful NF-kappaB inhibitor in myelodysplastic cells and in leukemia cell lines acting independently from cell iron deprivation by chelation and reactive oxygen species scavenging. Haematologica. 2010. 95:1308–1316.
Article13. Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004. 6:203–208.14. Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998. 8:297–303.
Article15. Zhang SQ, Kovalenko A, Cantarella G, Wallach D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKgamma) upon receptor stimulation. Immunity. 2000. 12:301–311.
Article16. Bubici C, Papa S, Pham CG, Zazzeroni F, Franzoso G. NF-kappaB and JNK: an intricate affair. Cell Cycle. 2004. 3:1524–1529.17. Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006. 25:6731–6748.
Article18. Guzman ML, Neering SJ, Upchurch D, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001. 98:2301–2307.
Article19. Ohyashiki JH, Kobayashi C, Hamamura R, Okabe S, Tauchi T, Ohyashiki K. The oral iron chelator deferasirox represses signaling through the mTOR in myeloid leukemia cells by enhancing expression of REDD1. Cancer Sci. 2009. 100:970–977.
Article20. Kim JL, Kang HN, Kang MH, Yoo YA, Kim JS, Choi CW. The oral iron chelator deferasirox induces apoptosis in myeloid leukemia cells by targeting caspase. Acta Haematol. 2011. 126:241–245.
Article21. Kontoghiorghes GJ, Eracleous E, Economides C, Kolnagou A. Advances in iron overload therapies. Prospects for effective use of deferiprone (L1), deferoxamine, the new experimental chelators ICL670, GT56-252, L1NA11 and their combinations. Curr Med Chem. 2005. 12:2663–2681.
Article22. Triantafyllou A, Liakos P, Tsakalof A, et al. The flavonoid quercetin induces hypoxia-inducible factor-1alpha (HIF-1alpha) and inhibits cell proliferation by depleting intracellular iron. Free Radic Res. 2007. 41:342–356.
Article23. Jung YS, Bae EY, Chung NG, et al. Comparison of immune responses induced by deferoxamine and deferasirox. Korean J Hematol. 2008. 43:150–158.
Article24. Richardson DR, Tran EH, Ponka P. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents. Blood. 1995. 86:4295–4306.
Article25. Borgna-Pignatti C, Cappellini MD, De Stefano P, et al. Cardiac morbidity and mortality in deferoxamine- or deferiprone-treated patients with thalassemia major. Blood. 2006. 107:3733–3737.
Article26. Valle P, Timeus F, Piglione M, et al. Effect of different exposures to desferrioxamine on neuroblastoma cell lines. Pediatr Hematol Oncol. 1995. 12:439–446.
Article27. Darnell G, Richardson DR. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents III: the effect of the ligands on molecular targets involved in proliferation. Blood. 1999. 94:781–792.
Article28. Kontoghiorghes GJ, Pattichi K, Hadjigavriel M, Kolnagou A. Transfusional iron overload and chelation therapy with deferoxamine and deferiprone (L1). Transfus Sci. 2000. 23:211–223.
Article29. Lescoat G, Chantrel-Groussard K, Pasdeloup N, Nick H, Brissot P, Gaboriau F. Antiproliferative and apoptotic effects in rat and human hepatoma cell cultures of the orally active iron chelator ICL670 compared to CP20: a possible relationship with polyamine metabolism. Cell Prolif. 2007. 40:755–767.
Article30. Chantrel-Groussard K, Gaboriau F, Pasdeloup N, et al. The new orally active iron chelator ICL670A exhibits a higher antiproliferative effect in human hepatocyte cultures than O-trensox. Eur J Pharmacol. 2006. 541:129–137.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Tubulointerstitial Nephritis Induced by Deferasirox following Hematopoietic Stem Cell Transplantation for Severe Aplastic Anemia
- Use of deferasirox, an iron chelator, to overcome imatinib resistance of chronic myeloid leukemia cells
- Diagnosis and treatment of transfusion-related iron overload
- Korean Guideline for Iron Chelation Therapy in Transfusion-Induced Iron Overload
- Economic Evaluation of Iron Chelation Agents: Oral Deferasirox versus Infusional Deferoxamine