J Korean Med Sci.
2004 Feb;19(1):123-126. 10.3346/jkms.2004.19.1.123.
X-linked Severe Combined Immunodeficiency Syndrome: The First Korean Case with gamma c Chain Gene Mutation and Subsequent Genetic Counseling
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Chungnam National University, Daejeon, Korea. hoonkook@chonnam.ac.kr
- 2Department of Pediatric Oncology, Institute of Development, Aging and Cancer Tohoku University, Sendai.
- 3Department of Pediatrics, Faculty of Medicine, Toyama Medical and Pharmaceutical University, Toyama, Japan.
- 4Department of Obstetrics, Chonnam National University Medical School, Gwangju, Korea.
- 5Department of Pediatrics, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 1785705
- DOI: http://doi.org/10.3346/jkms.2004.19.1.123
Abstract
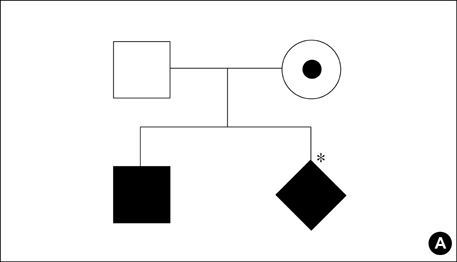
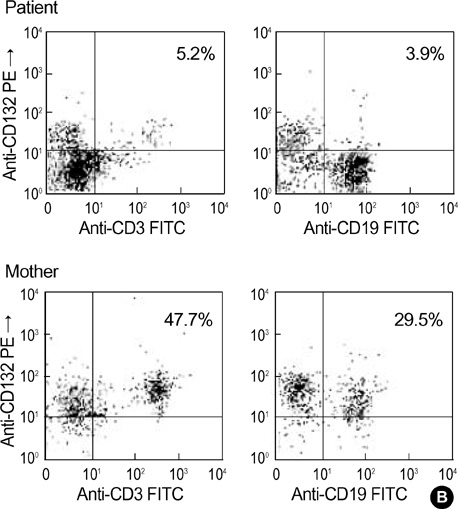
- X-linked severe combined immunodeficiency (X-SCID) is a rare, life-threatening immune disorder, caused by mutations in the gamma c chain gene, which encodes an essential component of the cytokine receptors for interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15, and IL-21. A 13-month-old boy with recurrent infections who had reduced serum immunoglobulin levels and decreased numbers of CD3, CD16/56 cells was evaluated for gamma c chain gene mutation and protein expression. The patient had a C-to-T point mutation at nucleotide position 690, one of the hot spots, resulting in a single amino acid substitution of cysteine for arginine (R226C), as determined by direct sequencing and PCR-RFLP. The patient's mother was a heterozygous carrier. Percutaneous umbilical cord blood sampling was performed at the 6-month of gestation in a subsequent pregnancy. As the immunophenotype of the fetus showed an identical pattern, the pregnancy was terminated and genetic analysis of the abortus confirmed recurrence. This is the first report of the molecular diagnosis of X-SCID in Korea. Genetic analysis of the gamma c chain gene is useful for definite diagnosis and genetic counseling for X-SCID.
Keyword
MeSH Terms
-
Arginine/chemistry
Cysteine/chemistry
DNA/metabolism
DNA Mutational Analysis
Female
Flow Cytometry
Genetic Counseling/*methods
Heterozygote
Human
Immunoglobulins/metabolism
Immunophenotyping/methods
Korea
*Linkage (Genetics)
Male
*Mutation
Pedigree
Point Mutation
Polymerase Chain Reaction
Polymorphism, Restriction Fragment Length
Receptors, Immunologic/*genetics
Sequence Analysis, DNA
Severe Combined Immunodeficiency/*diagnosis/*genetics
Support, Non-U.S. Gov't
Time Factors
*X Chromosome
Figure
Reference
-
1. Stephan JL, Vlekova V, Le Deist F, Blanche S, Donadieu J, De Saint-Basile G, Durandy A, Griscelli C, Fischer A. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 patients. J Pediatr. 1993. 123:564–572.
Article2. Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. The interleukin-2 receptor γchain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996. 14:179–205.3. Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993. 73:147–157.4. Puck JM, Deschenes SM, Porter JC, Dutra AS, Brown CJ, Willard HF, Henthorn PS. The interleukin-2 receptor gamma chain maps to Xq13.1 and is mutated in X-linked severe combined immunodeficiency, SCIDX1. Hum Mol Genet. 1993. 2:1099–1104.5. Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, Sugamura K. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001. 167:1–5.6. Takeshita T, Asao H, Ohtani K, Ishii N, Kumaki S, Tanaka N, Munakata H, Nakamura M, Sugamura K. Cloning of the γchain of the human IL-2 receptor. Science. 1992. 257:379–382.7. Kumaki S, Kondo M, Takeshita T, Asao H, Nakamura M, Sugamura K. Cloning of the mouse interleukin 2 receptor gamma chain: demonstration of functional differences between the mouse and human receptors. Biochem Biophys Res Commun. 1993. 193:356–363.8. Russell SM, Johnston JA, Noguchi M, Kawamura M, Bacon CM, Friedmann M, Berg M, McVicar DW, Witthuhn BA, Silvennoinen O, Goldman AS, Schmalstieg FC, Ihle JN, O'Shea JJ, Leonard WJ. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994. 266:1042–1045.
Article9. Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu Z-J, Oishi I, Silvennoinen O, Witthuhn BA, Ihle JN, Taniguchi T. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994. 266:1045–1047.
Article10. Kumaki S, Ishii N, Minegishi M, Ohashi Y, Hakozaki I, Nonoyama S, Imai K, Morio T, Tsuge I, Sakiyama Y, Miyanoshita A, Miura J, Mayumi M, Heike T, Katamura K, Takada H, Izumi I, Kamizono J, Hibi S, Sasaki H, Kimura M, Kikuta A, Date Y, Sako M, Tanaka H, Sano K, Sugamura K, Tsuchiya S. Characterization of the gamma c chain among 27 unrelated Japanese patients with X-linked severe combined immunodeficiency (X-SCID). Hum Genet. 2000. 107:406–408.11. Puck JM, Pepper AE, Henthorn PS, Candotti F, Isakov J, Whitwam T, Conley ME, Fischer RE, Rosenblatt HM, Small TN, Buckley RH. Mutation analysis of IL2RG in human X-linked severe combined immunodeficiency. Blood. 1997. 89:1968–1977.12. Kumaki S, Ochs HD, Timour M, Schooley K, Ahdieh M, Hill H, Sugamura K, Anderson D, Zhu Q, Cosman D, Giri JG. Characterization of B-cell lines established from two X-linked severe combined immunodeficiency patients: interleukin-15 binds to the B cells but is not internalized efficiently. Blood. 1995. 86:1428–1436.
Article13. Pepper AE, Buckley RH, Small TN, Puck JM. Two mutational hotspots in the interleukin-2 receptor gamma chain gene causing human X-linked severe combined immunodeficiency. Am J Hum Genet. 1995. 57:564–571.14. DiSanto JP, Dautry-Varsat A, Certain S, Fischer A, de Saint Basile G. Interleukin-2 (IL-2) receptor gamma chain mutations in X-linked severe combined immunodeficiency disease result in the loss of high-affinity IL-2 receptor binding. Eur J Immunol. 1994. 24:475–479.15. Ting SS, Leigh D, Lindeman R, Ziegler JB. Identification of X-linked severe combined immunodeficiency by mutation analysis of blood and hair roots. Br J Haematol. 1999. 106:190–194.
Article16. Ishii N, Asao H, Kimura Y, Takeshita T, Nakamura M, Tsuchiya S, Konno T, Maeda M, Uchiyama T, Sugamura K. Impairment of ligand binding and growth signaling of mutant IL-2 receptor gamma-chains in patients with X-linked severe combined immunodeficiency. J Immunol. 1994. 153:1310–1317.17. Conley ME. Molecular approaches to analysis of X-linked immunodeficiencies. Ann Rev Immunol. 1992. 10:215–238.
Article18. Macchi P, Villa A, Giliani S, Sacco MG, Frattini A, Porta F, Ugazio AG, Johnston JA, Candotti F, O'Shea JJ, Vezzoni P, Notarangelo LD. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature. 1995. 377:65–68.
Article19. von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995. 181:1519–1526.
Article20. Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994. 180:1955–1960.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mutation Analysis in X-linked Recessive Congenital Immunodeficiency Syndromes
- Effect of interferon-gamma treatment on interstitial pneumonia in a patient with severe combined immunodeficiency
- A Familial Case of Wiskott-Aldrich Syndrome with a Hotspot Mutation in Exon 2 of the WAS Gene
- Genetic Counseling Can Influence the Course of a Suspected Familial Cancer Syndrome Patient: From a Case of Li-Fraumeni Like Syndrome with a Germline Mutation in the TP53 Gene
- A novel mutation in XLRS1 gene in X-linked juvenile retinoschisis