J Periodontal Implant Sci.
2011 Jun;41(3):149-156. 10.5051/jpis.2011.41.3.149.
Effect of globular adiponectin on interleukin-6 and interleukin-8 expression in periodontal ligament and gingival fibroblasts
- Affiliations
-
- 1Department of Oral Biology, BK21 Project, Oral Science Research Center, Yonsei University College of Dentistry, Seoul, Korea. yu618@yuhs.ac
- 2Oral Cancer Research Institute, Yonsei University College of Dentistry, Seoul, Korea.
- 3Research Center for Orofacial Hard Tissue Regeneration, Yonsei University College of Dentistry, Seoul, Korea.
- 4Department of Applied Life Science, Yonsei University Graduate School, Seoul, Korea.
- 5Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea.
- KMID: 1783606
- DOI: http://doi.org/10.5051/jpis.2011.41.3.149
Abstract
- PURPOSE
Globular adiponectin (gAd) is a type of adipocytokine, which is mainly produced by adipose tissue. It has been reported that gAd acts as a pro- as well as an anti-inflammatory factor. Interleukin (IL)-6 and IL-8 are pro-inflammatory cytokines. To investigate the role of gAd on periodontal tissues, the expression of adiponectin receptor 1 (AdipoR1) and the effect of gAd on the expression of IL-6 and IL-8 were investigated in periodontal ligament (PDL) and gingival fibroblasts.
METHODS
PDL and gingival fibroblasts were cultured from human periodontal tissues. gAd derived from Escherichia coli and murine myeloma cells were used. The expression of AdipoR1 was estimated by reverse transcription-polymerase chain reaction and western blot. The expression of cytokines was measured by enzyme-linked immunosorbent assay.
RESULTS
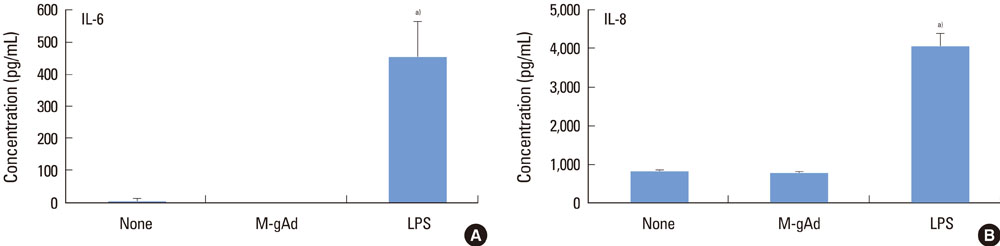
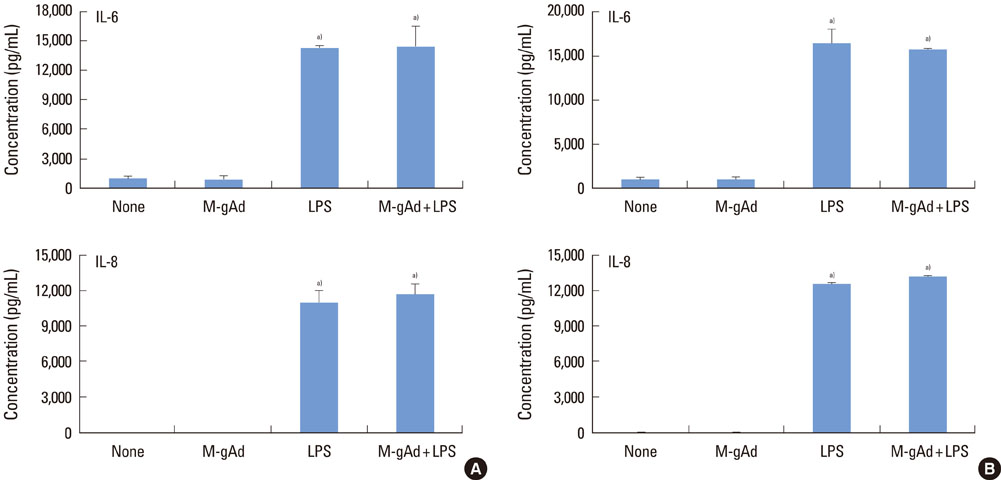
PDL and gingival fibroblasts expressed both mRNA and protein of AdipoR1. gAd derived from E. coli increased the production of IL-6 and IL-8, but polymyxin B, an inhibitor of lipopolysaccharide (LPS), inhibited IL-6 and IL-8 production induced by gAd in both types of cells. gAd derived from murine myeloma cells did not induce IL-6 and IL-8 production in those cells. gAd derived from E. coli contained higher levels of LPS than gAd derived from murine myeloma cells. LPS increased production of IL-6 and IL-8 in PDL and gingival fibroblasts, but pretreatment of cells with gAd derived from murine myeloma cells did not inhibit LPS-induced IL-6 and IL-8 expression.
CONCLUSIONS
Our results suggest that PDL and gingival fibroblasts express AdipoR1 and that gAd does not act as a modulator of IL-6 and IL-8 expression in PDL and gingival fibroblasts.
Keyword
MeSH Terms
Figure
Reference
-
1. Ekuni D, Yamamoto T, Koyama R, Tsuneishi M, Naito K, Tobe K. Relationship between body mass index and periodontitis in young Japanese adults. J Periodontal Res. 2008. 43:417–421.
Article2. Han DH, Lim SY, Sun BC, Paek DM, Kim HD. Visceral fat area-defined obesity and periodontitis among Koreans. J Clin Periodontol. 2010. 37:172–179.
Article3. Mealey BL, Rose LF. Diabetes mellitus and inflammatory periodontal diseases. Curr Opin Endocrinol Diabetes Obes. 2008. 15:135–141.
Article4. Sun Y, Xun K, Wang C, Zhao H, Bi H, Chen X, et al. Adiponectin, an unlocking adipocytokine. Cardiovasc Ther. 2009. 27:59–75.
Article5. Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance. J Clin Endocrinol Metab. 2004. 89:447–452.
Article6. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006. 6:772–783.
Article7. Lago F, Dieguez C, Gómez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007. 3:716–724.
Article8. Huang D, Yang C, Wang Y, Liao Y, Huang K. PARP-1 suppresses adiponectin expression through poly(ADP-ribosyl) ation of PPAR gamma in cardiac fibroblasts. Cardiovasc Res. 2009. 81:98–107.
Article9. Tan W, Wang F, Zhang M, Guo D, Zhang Q, He S. High adiponectin and adiponectin receptor 1 expression in synovial fluids and synovial tissues of patients with rheumatoid arthritis. Semin Arthritis Rheum. 2009. 38:420–427.
Article10. Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003. 423:762–769.
Article11. Rovin BH, Song H. Chemokine induction by the adipocyte-derived cytokine adiponectin. Clin Immunol. 2006. 120:99–105.
Article12. Tang CH, Chiu YC, Tan TW, Yang RS, Fu WM. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-kappa B pathway. J Immunol. 2007. 179:5483–5492.
Article13. Kitahara K, Kusunoki N, Kakiuchi T, Suguro T, Kawai S. Adiponectin stimulates IL-8 production by rheumatoid synovial fibroblasts. Biochem Biophys Res Commun. 2009. 378:218–223.
Article14. Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, et al. Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem Biophys Res Commun. 2005. 335:1254–1263.
Article15. Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, et al. Peripheral factors in the metabolic syndrome: the pivotal role of adiponectin. Ann N Y Acad Sci. 2006. 1083:185–195.
Article16. Liao W, Yu C, Wen J, Jia W, Li G, Ke Y, et al. Adiponectin induces interleukin-6 production and activates STAT3 in adult mouse cardiac fibroblasts. Biol Cell. 2009. 101:263–272.
Article17. Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000. 96:1723–1732.
Article18. Neumeier M, Weigert J, Schäffler A, Wehrwein G, Müller-Ladner U, Schölmerich J, et al. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006. 79:803–808.
Article19. Turner JJ, Smolinska MJ, Sacre SM, Foxwell BM. Induction of TLR tolerance in human macrophages by adiponectin: does LPS play a role? Scand J Immunol. 2009. 69:329–336.
Article20. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999. 257:79–83.
Article21. Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000. 20:1595–1599.
Article22. Furugen R, Hayashida H, Yamaguchi N, Yoshihara A, Ogawa H, Miyazaki H, et al. The relationship between periodontal condition and serum levels of resistin and adiponectin in elderly Japanese. J Periodontal Res. 2008. 43:556–562.
Article23. Nishihara R, Sugano N, Takano M, Shimada T, Tanaka H, Oka S, et al. The effect of Porphyromonas gingivalis infection on cytokine levels in type 2 diabetic mice. J Periodontal Res. 2009. 44:305–310.
Article24. Matsumoto S, Ogawa H, Soda S, Hirayama S, Amarasena N, Aizawa Y, et al. Effect of antimicrobial periodontal treatment and maintenance on serum adiponectin in type 2 diabetes mellitus. J Clin Periodontol. 2009. 36:142–148.
Article25. Kamio N, Akifusa S, Yamaguchi N, Nonaka K, Yamashita Y. Anti-inflammatory activity of a globular adiponectin function on RAW 264 cells stimulated by lipopolysaccharide from Aggregatibacter actinomycetemcomitans. FEMS Immunol Med Microbiol. 2009. 56:241–247.
Article26. Yamaguchi N, Kukita T, Li YJ, Martinez Argueta JG, Saito T, Hanazawa S, et al. Adiponectin inhibits osteoclast formation stimulated by lipopolysaccharide from Actinobacillus actinomycetemcomitans. FEMS Immunol Med Microbiol. 2007. 49:28–34.
Article27. Scheres N, Laine ML, de Vries TJ, Everts V, van Winkelhoff AJ. Gingival and periodontal ligament fibroblasts differ in their inflammatory response to viable Porphyromonas gingivalis. J Periodontal Res. 2010. 45:262–270.
Article28. Lee YS, Bak EJ, Kim M, Park W, Seo JT, Yoo YJ. Induction of IL-8 in periodontal ligament cells by H(2)O (2). J Microbiol. 2008. 46:579–584.29. Chinetti G, Zawadski C, Fruchart JC, Staels B. Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors PPARalpha, PPARgamma, and LXR. Biochem Biophys Res Commun. 2004. 314:151–158.
Article30. Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y, et al. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep. 2008. 20:971–977.
Article31. Ehling A, Schäffler A, Herfarth H, Tarner IH, Anders S, Distler O, et al. The potential of adiponectin in driving arthritis. J Immunol. 2006. 176:4468–4478.
Article32. Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998. 9:248–266.
Article33. Preshaw PM, Taylor JJ. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J Clin Periodontol. 2011. 38:Suppl 11. 60–84.
Article34. Saito T, Yamaguchi N, Shimazaki Y, Hayashida H, Yonemoto K, Doi Y, et al. Serum levels of resistin and adiponectin in women with periodontitis: the Hisayama study. J Dent Res. 2008. 87:319–322.
Article35. Yamaguchi N, Hamachi T, Kamio N, Akifusa S, Masuda K, Nakamura Y, et al. Expression levels of adiponectin receptors and periodontitis. J Periodontal Res. 2010. 45:296–300.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Induction of IL-6 and IL-8 Expression by Leptin Treatment in Periodontal Ligament Cells and Gingival Fibroblasts
- Effects of mechanical stress and interleukin-1beta on collagenase and TIMP-1 expression in human periodontal ligament fibroblasts
- Effect of various cytokines on the production of prostaglandin E2 leukotriene B4 and collagenase in human periodontal ligament fibroblasts in vitro
- Biological Characteristics of Human Periodontal Ligament Cells
- Effect of Glucose and Insulin on Human Gingival Fibroblasts and Periodontal Ligament Cells