J Vet Sci.
2013 Dec;14(4):387-393. 10.4142/jvs.2013.14.4.387.
Chemopreventive and metabolic effects of inulin on colon cancer development
- Affiliations
-
- 1Institute of Experimental Medicine, Faculty of Medicine, Pavol Jozef Safarik University, Kosice 040 11, The Slovak Republic. emilia.hijova@upjs.sk
- 2Institute of Parasitology of the Slovak Academy of Sciences, Kosice 040 01, The Slovak Republic.
- KMID: 1712304
- DOI: http://doi.org/10.4142/jvs.2013.14.4.387
Abstract
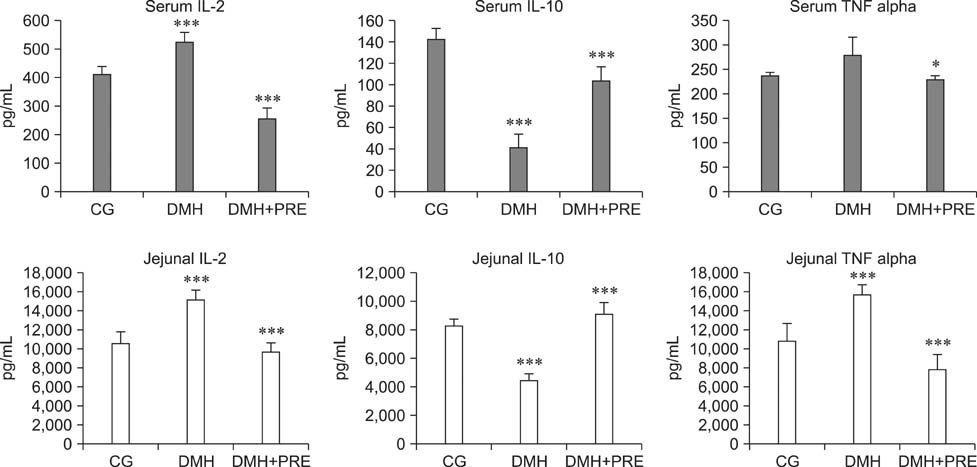
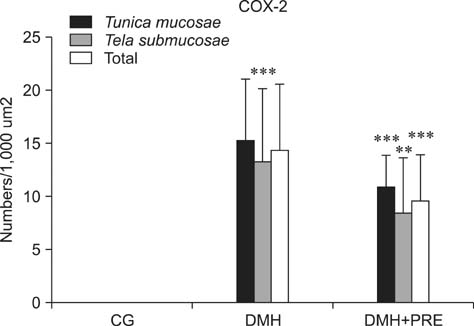
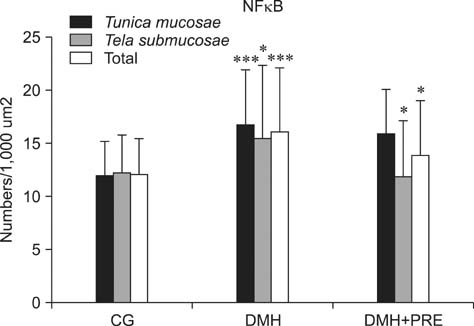
- Prebiotics modulate microbial composition and ensure a healthy gastrointestinal tract environment that can prevent colon cancer development. These natural dietary compounds are therefore potential chemopreventive agents. Thirty Sprague-Dawley rats (4 months old) were experimentally treated with procarcinogen dimethylhydrazine to induce colon cancer development. The rats were randomly assigned to three groups: a control group (CG), a group treated with dimethylhydrazine (DMH), and a group given DMH and inulin, a prebiotic (DMH+PRE). The effects of inulin on the activities of bacterial glycolytic enzymes, short-chain fatty acids, coliform and lactobacilli counts, cytokine levels, and cyclooxygenase-2 (COX-2) and transcription nuclear factor kappa beta (NFkappaB) immunoreactivity were measured. Inulin significantly decreased coliform counts (p < 0.01), increased lactobacilli counts (p < 0.001), and decreased the activity of beta-glucuronidase (p < 0.01). Butyric and propionic concentrations were decreased in the DMH group. Inulin increased its concentration that had been reduced by DMH. Inulin decreased the numbers of COX-2- and NFkappaB-positive cells in the tunica mucosae and tela submucosae of the colon. The expression of IL-2, TNFalpha, and IL-10 was also diminished. This 28-week study showed that dietary intake of inulin prevents preneoplastic changes and inflammation that promote colon cancer development.
Keyword
MeSH Terms
-
Animals
Bacterial Proteins/genetics/metabolism
Colon/enzymology
Colonic Neoplasms/chemically induced/*drug therapy/metabolism
Colony Count, Microbial
Cyclooxygenase 2/genetics/metabolism
Cytokines/blood/genetics
Diet
Dietary Supplements/analysis
Dimethylhydrazines/toxicity
Enterobacteriaceae/drug effects/physiology
Fatty Acids, Volatile/genetics/metabolism
Female
Gene Expression Regulation/drug effects
Inulin/administration & dosage/*metabolism
Lactobacillaceae/drug effects/physiology
Male
NF-kappa B/genetics/metabolism
Prebiotics/*analysis
Rats
Rats, Sprague-Dawley
Bacterial Proteins
Cytokines
Cyclooxygenase 2
Dimethylhydrazines
Fatty Acids, Volatile
Inulin
NF-kappa B
Prebiotics
Figure
Reference
-
1. Bakhle YS. COX-2 and cancer: a new approach to an old problem. Br J Pharmacol. 2001; 134:1137–1150.
Article2. Benson AB 3rd. Epidemiology, disease progression, and economic burden of colorectal cancer. J Manag Care Pharm. 2007; 13:6 Suppl C. S5–S18.
Article3. Comalada M, Bailón E, de Haro O, Lara-Villoslada F, Xaus J, Zarzuelo A, Gálvez J. The effects of short-chain fatty acids on colo epithelial proliferation and survival depend on the cellular phenotype. J Cancer Res Clin Oncol. 2006; 132:487–497.
Article4. Chabot-Fletcher M. Transcription factor NFκB: an emerging anti-inflammatory drug target. Pharmacol Commun. 1996; 8:317–324.5. Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-κB, a ubiquitous transcription factor in the iniciation of diseases. Clin Chem. 1999; 45:7–17.
Article6. Femia AP, Salvadori M, Broekaert WF, François IEJA, Delcour JA, Courtin CM, Caderni G. Arabinoxylan-oligosaccharides (AXOS) reduce preneoplastic lesions in the colon of rats treated with 1,2-dimethylhydrazine (DMH). Eur J Nutr. 2010; 49:127–132.
Article7. Galisteo M, Duarte J, Zarzuelo A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J Nutr Biochem. 2008; 19:71–84.
Article8. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995; 125:1401–1412.
Article9. Gibson GR, Probert HM, Loo VJ, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004; 17:259–275.
Article10. Henriques VT, Dias CMGC, Franceschini SCC, Sabarense CM, Costa NMB, Leite JIA, Peluzio MCG. Omega-3 fatty acids reduce the development of preneoplatic lesions. Rev Nutr Campinas. 2009; 22:237–244.11. Janakiram NB, Rao CV. Molecular markers and targets for colorectal cancer prevention. Acta Pharmacol Sin. 2008; 29:1–20.
Article12. Juskiewicz J, Zdunczyk Z, Wroblewska M, Oszmianski J, Hernandez T. The responce of rats to feeding with diets. Food Res Int. 2002; 35:201–205.13. Juśkiewicz J, Wróblewska M, Jaroslawska J, Baliński P, Matusevičius P, Zduńczyk P, Biedrzycka E, Zduńczyk Z. Effects of inulin supplemented to cellulose-free or cellulose-rich diets on caecal environment and biochemical blood parameters in rats. J Anim Feed Sci. 2009; 18:709–722.
Article14. Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acids in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta. 2010; 1801:1175–1183.
Article15. Lay C, Sutren M, Lepercq P, Juste C, Rigottier-Gois L, Lhoste E, Lemée R, Le Ruyet P, Doré J, Andrieux C. Influence of Camembert consumption on the composition and metabolism of intestinal microbiota: A study in human microbiota-associated rats. Br J Nutr. 2004; 92:429–438.
Article16. Mathew A, Peters U, Chatterjee N, Kulldorff M, Sinha R. Fat, fiber, fruits, vegetables, and risk of colorectal adenomas. Int J Cancer. 2004; 108:287–292.
Article17. Pan MH, Ho CT. Chemopreventive effects of natural dietary compounds on cancer development. Chem Soc Rev. 2008; 37:2558–2574.
Article18. Pool-Zobel BL, Sauer J. Overview of experimental data on reduction of colorectal cancer risk by inulin-type fructans. J Nutr. 2007; 137:2580S–2584S.
Article19. Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995; 55:3785–3789.20. Scharlau D, Borowicki A, Habermann N, Hofmann T, Klenow S, Miene C, Munjal U, Stein K, Glei M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat Res. 2009; 682:39–53.
Article21. Schley PD, Field CJ. The immune-enhancing effect of dietary fibres and prebiotics. Br J Nutr. 2002; 87:Suppl 2. S221–S230.22. Versteeg HH, van Bergen en Henegouwen PM, van Deventer SJH, Peppelenbosch MP. Cyclooxygenase-dependent signalling: molecular events and consequences. FEBS Lett. 1999; 445:1–5.
Article23. Wong JMW, de Souza R, Kendall CWC, Emam A, Jenkins DJA. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006; 40:235–243.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Studies on the Renal Inulin Clearance and Inulin Space in Post-hemorrhagic Anemic Men
- Chemopreventive and Chemotherapeutic Effects of Fish Oil derived Omega-3 Polyunsaturated Fatty Acids on Colon Carcinogenesis
- The role of microbiome in colorectal carcinogenesis and its clinical potential as a target for cancer treatment
- Systemic Therapy for Advanced and Metastatic Colon Cancer
- Effects of Jerusalem Artichoke Extract and Inulin on Blood Glucose Levels and Insulin Secretion in Streptozotocin Induced Diabetic Mice