Intest Res.
2022 Jan;20(1):31-42. 10.5217/ir.2021.00034.
The role of microbiome in colorectal carcinogenesis and its clinical potential as a target for cancer treatment
- Affiliations
-
- 1Department of Internal Medicine, Dongguk University Ilsan Hospital, Dongguk University College of Medicine, Goyang, Korea
- KMID: 2525070
- DOI: http://doi.org/10.5217/ir.2021.00034
Abstract
- The role of gut microbiome-intestinal immune complex in the development of colorectal cancer and its progression is well recognized. Accordingly, certain microbial strains tend to colonize or vanish in patients with colorectal cancer. Probiotics, prebiotics, and synbiotics are expected to exhibit both anti-tumor effects and chemopreventive effects during cancer treatment through mechanisms such as xenometabolism, immune interactions, and altered eco-community. Microbial modulation can also be safely used to prevent complications during peri-operational periods of colorectal surgery. A deeper understanding of the role of intestinal microbiota as a target for colorectal cancer treatment will lead the way to a better prognosis for colorectal cancer patients.
Figure
Cited by 3 articles
-
Calcium, Vitamin D, and Colorectal Cancer
Young-Jo Wi, Soo-Young Na
Korean J Gastroenterol. 2023;82(2):47-55. doi: 10.4166/kjg.2023.091.식이가 대장암의 진행 및 예방에 미치는 영향: 영양소부터 종양 발생까지
Sang Hoon Kim, Dong Hwan Park, Yun Jeong Lim
Korean J Gastroenterol. 2023;82(2):73-83. doi: 10.4166/kjg.2023.079.Gut Microbiome and Colorectal Cancer
Tae-Geun Gweon
Korean J Gastroenterol. 2023;82(2):56-62. doi: 10.4166/kjg.2023.089.
Reference
-
1. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019; Sep. 69:363–385.2. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017; 67:177–193.
Article3. Miller K. Excellent care for cancer survivors: a guide to fully meet their needs in medical offices and in the community. Santa Barbara: ABC-CLIO;2012.4. Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016; 164:337–340.5. Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011; 474:327–336.6. Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012; 336:1262–1267.
Article7. Wilson ID, Nicholson JK. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res. 2017; 179:204–222.
Article8. Barko PC, McMichael MA, Swanson KS, Williams DA. The gastrointestinal microbiome: a review. J Vet Intern Med. 2018; 32:9–25.9. Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001; 2:361–367.
Article10. Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006; 36:2336–2346.11. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008; 133:775–787.
Article12. Josefowicz SZ, Niec RE, Kim HY, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012; 482:395–399.
Article13. Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005; 115:1923–1933.
Article14. Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008; 453:620–625.
Article15. Round JL, Lee SM, Li J, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011; 332:974–977.
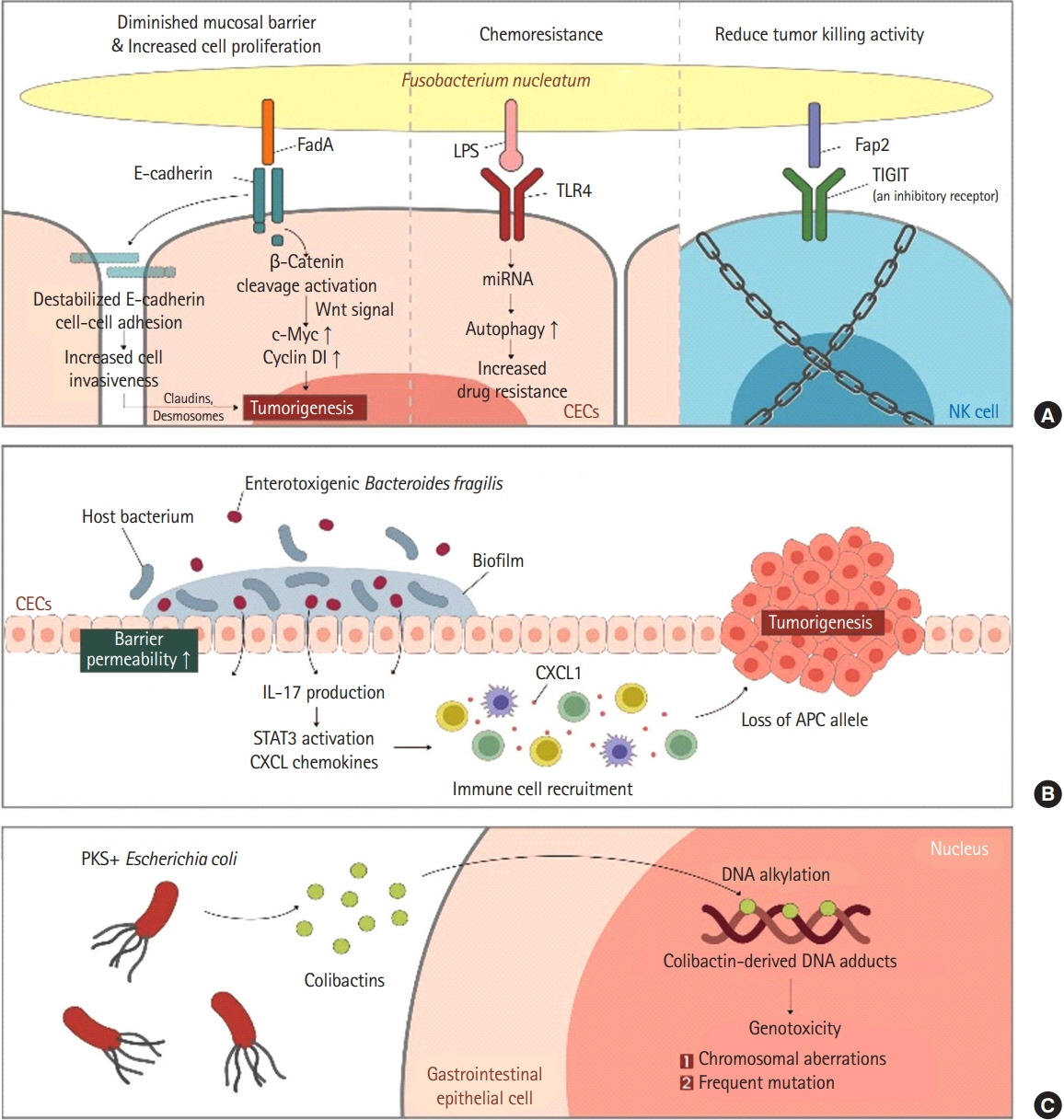
Article16. Rabizadeh S, Rhee KJ, Wu S, et al. Enterotoxigenic Bacteroides fragilis: a potential instigator of colitis. Inflamm Bowel Dis. 2007; 13:1475–1483.
Article17. Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009; 15:1016–1022.18. Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005; 201:233–240.
Article19. Zhang GX, Gran B, Yu S, et al. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003; 170:2153–2160.
Article20. Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008; 112:2340–2352.
Article21. Osorio F, LeibundGut-Landmann S, Lochner M, et al. DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol. 2008; 38:3274–3281.
Article22. Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007; 178:6725–6729.
Article23. Yang XO, Nurieva R, Martinez GJ, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008; 29:44–56.
Article24. Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009; 31:677–689.
Article25. Wang Y, Yin Y, Chen X, et al. Induction of intestinal Th17 cells by flagellins from segmented filamentous bacteria. Front Immunol. 2019; 10:2750.26. Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987; 28:1221–1227.
Article27. Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013; 504:446–450.
Article28. McDermott AJ, Huffnagle GB. The microbiome and regulation of mucosal immunity. Immunology. 2014; 142:24–31.29. Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol. 2005; 174:3237–3246.
Article30. Feleszko W, Jaworska J, Rha RD, et al. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007; 37:498–505.31. Chen GY. The role of the gut microbiome in colorectal cancer. Clin Colon Rectal Surg. 2018; 31:192–198.
Article32. Park CH, Eun CS, Han DS. Intestinal microbiota, chronic inflammation, and colorectal cancer. Intest Res. 2018; 16:338–345.
Article33. Gagnière J, Raisch J, Veziant J, et al. Gut microbiota imbalance and colorectal cancer. World J Gastroenterol. 2016; 22:501–518.34. Yu LC, Wei SC, Ni YH. Impact of microbiota in colorectal carcinogenesis: lessons from experimental models. Intest Res. 2018; 16:346–357.
Article35. Feng Q, Liang S, Jia H, et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015; 6:6528.
Article36. Arthur JC, Perez-Chanona E, Mühlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012; 338:120–123.
Article37. Purcell RV, Pearson J, Aitchison A, Dixon L, Frizelle FA, Keenan JI. Colonization with enterotoxigenic Bacteroides fragilis is associated with early-stage colorectal neoplasia. PLoS One. 2017; 12:e0171602.
Article38. Bonnet M, Buc E, Sauvanet P, et al. Colonization of the human gut by E. coli and colorectal cancer risk. Clin Cancer Res. 2014; 20:859–867.39. Raisch J, Buc E, Bonnet M, et al. Colon cancer-associated B2 Escherichia coli colonize gut mucosa and promote cell proliferation. World J Gastroenterol. 2014; 20:6560–6572.
Article40. Nougayrède JP, Homburg S, Taieb F, et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006; 313:848–851.41. Secher T, Samba-Louaka A, Oswald E, Nougayrède JP. Escherichia coli producing colibactin triggers premature and transmissible senescence in mammalian cells. PLoS One. 2013; 8:e77157.
Article42. Cougnoux A, Dalmasso G, Martinez R, et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut. 2014; 63:1932–1942.
Article43. Marchès O, Ledger TN, Boury M, et al. Enteropathogenic and enterohaemorrhagic Escherichia coli deliver a novel effector called Cif, which blocks cell cycle G2/M transition. Mol Microbiol. 2003; 50:1553–1567.
Article44. Maddocks OD, Short AJ, Donnenberg MS, Bader S, Harrison DJ. Attaching and effacing Escherichia coli downregulate DNA mismatch repair protein in vitro and are associated with colorectal adenocarcinomas in humans. PLoS One. 2009; 4:e5517.45. Nowrouzian FL, Oswald E. Escherichia coli strains with the capacity for long-term persistence in the bowel microbiota carry the potentially genotoxic pks island. Microb Pathog. 2012; 53:180–182.46. Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrède JP. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci U S A. 2010; 107:11537–115342.47. Hussan H, Clinton SK, Roberts K, Bailey MT. Fusobacterium’s link to colorectal neoplasia sequenced: a systematic review and future insights. World J Gastroenterol. 2017; 23:8626–8650.
Article48. Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013; 14:207–215.
Article49. Bachrach G, Ianculovici C, Naor R, Weiss EI. Fluorescence based measurements of Fusobacterium nucleatum coaggregation and of fusobacterial attachment to mammalian cells. FEMS Microbiol Lett. 2005; 248:235–240.50. Gur C, Ibrahim Y, Isaacson B, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015; 42:344–355.51. Mima K, Nishihara R, Qian ZR, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016; 65:1973–1980.
Article52. Brennan CA, Garrett WS. Fusobacterium nucleatum: symbiont, opportunist and oncobacterium. Nat Rev Microbiol. 2019; 17:156–166.53. Sánchez B, Delgado S, Blanco-Míguez A, Lourenço A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. 2017; 61:1600240.54. Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 2017; 14:356–365.
Article55. Heydari Z, Rahaie M, Alizadeh AM, Agah S, Khalighfard S, Bahmani S. Effects of Lactobacillus acidophilus and Bifidobacterium bifidum probiotics on the expression of microRNAs 135b, 26b, 18a and 155, and their involving genes in mice colon cancer. Probiotics Antimicrob Proteins. 2019; 11:1155–1162.
Article56. Baldwin C, Millette M, Oth D, Ruiz MT, Luquet FM, Lacroix M. Probiotic Lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutr Cancer. 2010; 62:371–378.57. Dos Reis SA, da Conceição LL, Siqueira NP, Rosa DD, da Silva LL, Peluzio MD. Review of the mechanisms of probiotic actions in the prevention of colorectal cancer. Nutr Res. 2017; 37:1–19.
Article58. Escamilla J, Lane MA, Maitin V. Cell-free supernatants from probiotic Lactobacillus casei and Lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutr Cancer. 2012; 64:871–878.
Article59. Soltan Dallal MM, Mojarrad M, Baghbani F, Raoofian R, Mardaneh J, Salehipour Z. Effects of probiotic Lactobacillus acidophilus and Lactobacillus casei on colorectal tumor cells activity (CaCo-2). Arch Iran Med. 2015; 18:167–172.60. An J, Ha EM. Combination therapy of Lactobacillus plantarum supernatant and 5-fluouracil increases chemosensitivity in colorectal cancer cells. J Microbiol Biotechnol. 2016; 26:1490–1503.
Article61. Saber A, Alipour B, Faghfoori Z, Mousavi Jam A, Yari Khosroushahi A. Secretion metabolites of probiotic yeast, Pichia kudriavzevii AS-12, induces apoptosis pathways in human colorectal cancer cell lines. Nutr Res. 2017; 41:36–46.
Article62. Mi H, Dong Y, Zhang B, et al. Bifidobacterium infantis ameliorates chemotherapy-induced intestinal mucositis via regulating T cell immunity in colorectal cancer rats. Cell Physiol Biochem. 2017; 42:2330–2341.
Article63. Osterlund P, Ruotsalainen T, Korpela R, et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer. 2007; 97:1028–1034.
Article64. Golkhalkhali B, Rajandram R, Paliany AS, et al. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: a randomized controlled trial. Asia Pac J Clin Oncol. 2018; 14:179–191.
Article65. Mego M, Chovanec J, Vochyanova-Andrezalova I, et al. Prevention of irinotecan induced diarrhea by probiotics: a randomized double blind, placebo controlled pilot study. Complement Ther Med. 2015; 23:356–362.
Article66. Aisu N, Tanimura S, Yamashita Y, et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Exp Ther Med. 2015; 10:966–972.
Article67. Yang Y, Xia Y, Chen H, et al. The effect of perioperative probiotics treatment for colorectal cancer: short-term outcomes of a randomized controlled trial. Oncotarget. 2016; 7:8432–8440.
Article68. Chen ZY, Hsieh YM, Huang CC, Tsai CC. Inhibitory effects of probiotic Lactobacillus on the growth of human colonic carcinoma cell line HT-29. Molecules. 2017; 22:107.
Article69. Martín R, Langella P. Emerging health concepts in the probiotics field: streamlining the definitions. Front Microbiol. 2019; 10:1047.
Article70. Chang CJ, Lin TL, Tsai YL, et al. Next generation probiotics in disease amelioration. J Food Drug Anal. 2019; 27:615–622.
Article71. Wang Y, Ma R, Liu F, Lee SA, Zhang L. Modulation of gut microbiota: a novel paradigm of enhancing the efficacy of programmed death-1 and programmed death ligand-1 blockade therapy. Front Immunol. 2018; 9:374.
Article72. Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol. 2017; 8:1765.
Article73. Guthrie L, Gupta S, Daily J, Kelly L. Human microbiome signatures of differential colorectal cancer drug metabolism. NPJ Biofilms Microbiomes. 2017; 3:27.
Article74. Wallace BD, Wang H, Lane KT, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010; 330:831–835.
Article75. Wang J, Feng W, Zhang S, et al. Gut microbial modulation in the treatment of chemotherapy-induced diarrhea with Shenzhu Capsule. BMC Complement Altern Med. 2019; 19:126.
Article76. Wang YH, Yao N, Wei KK, et al. The efficacy and safety of probiotics for prevention of chemoradiotherapy-induced diarrhea in people with abdominal and pelvic cancer: a systematic review and meta-analysis. Eur J Clin Nutr. 2016; 70:1246–1253.
Article77. Serna-Thomé G, Castro-Eguiluz D, Fuchs-Tarlovsky V, et al. Use of functional foods and oral supplements as adjuvants in cancer treatment. Rev Invest Clin. 2018; 70:136–146.
Article78. Tan CK, Said S, Rajandram R, Wang Z, Roslani AC, Chin KF. Pre-surgical administration of microbial cell preparation in colorectal cancer patients: a randomized controlled trial. World J Surg. 2016; 40:1985–1992.
Article79. Kotzampassi K, Stavrou G, Damoraki G, et al. A four-probiotics regimen reduces postoperative complications after colorectal surgery: a randomized, double-blind, placebo-controlled study. World J Surg. 2015; 39:2776–2783.
Article80. Liu ZH, Huang MJ, Zhang XW, et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: a double-center and double-blind randomized clinical trial. Am J Clin Nutr. 2013; 97:117–126.
Article81. Xie X, He Y, Li H, et al. Effects of prebiotics on immunologic indicators and intestinal microbiota structure in perioperative colorectal cancer patients. Nutrition. 2019; 61:132–142.
Article82. Bruno-Barcena JM, Azcarate-Peril MA. Galacto-oligosaccharides and colorectal cancer: feeding our intestinal probiome. J Funct Foods. 2015; 12:92–108.
Article83. Kuugbee ED, Shang X, Gamallat Y, et al. Structural change in microbiota by a probiotic cocktail enhances the gut barrier and reduces cancer via TLR2 signaling in a rat model of colon cancer. Dig Dis Sci. 2016; 61:2908–2920.
Article84. Saito Y, Hinoi T, Adachi T, et al. Synbiotics suppress colitis-induced tumorigenesis in a colon-specific cancer mouse model. PLoS One. 2019; 14:e0216393.
Article85. Rafter J, Bennett M, Caderni G, et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr. 2007; 85:488–496.
Article86. de Moura NA, Caetano BF, Sivieri K, et al. Protective effects of yacon (Smallanthus sonchifolius) intake on experimental colon carcinogenesis. Food Chem Toxicol. 2012; 50:2902–2910.
Article87. Gavresea F, Vagianos C, Korontzi M, et al. Beneficial effect of synbiotics on experimental colon cancer in rats. Turk J Gastroenterol. 2018; 29:494–501.
Article88. Li Y, Elmén L, Segota I, et al. Prebiotic-induced anti-tumor immunity attenuates tumor growth. Cell Rep. 2020; 30:1753–1766.
Article89. Lee CW, Chen HJ, Chien YH, Hsia SM, Chen JH, Shih CK. Synbiotic combination of djulis (Chenopodium formosanum) and Lactobacillus acidophilus inhibits colon carcinogenesis in rats. Nutrients. 2019; 12:103.
Article90. Krebs B. Prebiotic and synbiotic treatment before colorectal surgery: randomised double blind trial. Coll Antropol. 2016; 40:35–40.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Crossroad between inflammation and carcinogenesis in colon
- Gut Microbiome and Colorectal Cancer
- Intestinal microbiota, chronic inflammation, and colorectal cancer
- Potential of the Microbiome as a Biomarker for Early Diagnosis and Prognosis of Breast Cancer
- Gut Microbial Influence and Probiotics on Colorectal Cancer