J Korean Soc Spine Surg.
2007 Mar;14(1):25-33. 10.4184/jkss.2007.14.1.25.
The Effect of Synovial Fluid from Degenerated Facet on Hypertrophy and Ossification of the Ligamentum Flavum
- Affiliations
-
- 1Department of Orthopedic Surgery, Yonsei University College of Medicine, Korea. shmoon@yumc.yonsei.ac.kr
- 2Institute for Biomaterial Research, Korea Bone Bank Co. Lt, Korea.
- 3Department of Orthopedic Surgery, Korea University College of Medicine, Korea.
- 4Department of Mechanical Engineering, Yonsei University, Korea.
- 5School of Advanced Materials Engineering, Yonsei University, Korea.
- KMID: 1527140
- DOI: http://doi.org/10.4184/jkss.2007.14.1.25
Abstract
-
STUDY DESIGN: In vitro experimental study
OBJECTIVES
To examine the effect of a synovial supernatant on the cell viability, osteogenic phenotype, mRNA expression of the types collagen and various transcriptional factors on osteogenesis in ligamentum flavum (LF) cells stimulated with synovial fluid from a degenerated facet joint. LITERATURE REVIEW: In degenerative lumbar spinal stenosis, hypertrophied LF or osteoarthritic hypertrophy of a facet joint often causes neurogenic claudication. The facet joint is a synovial joint with hyaline cartilage on each side. Therefore, osteoarthritis of a facet joint eventually occurs with aging and other degenerative conditions of the spine. In lumbar spinal degeneration, inflammatory mediators or cytokines are released from the facet joint tissue, which consequently affects the adjacent LF because the LF covers posterolateral aspect of the spinal canal near facet joints. However, there are no reports on the relationship between a degenerated facet joint fluid and the LF in the lumbar spine.
MATERIALS AND METHODS
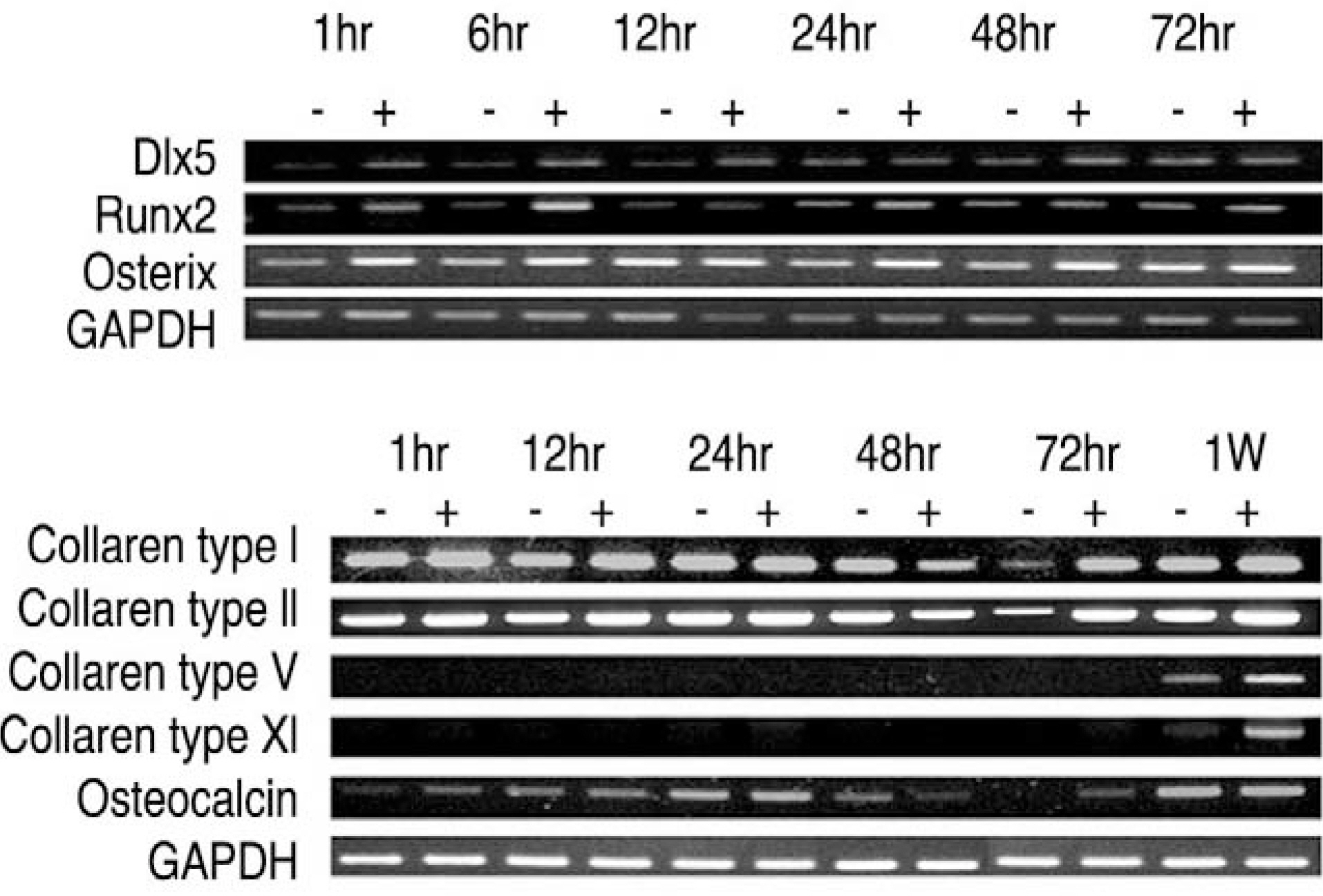
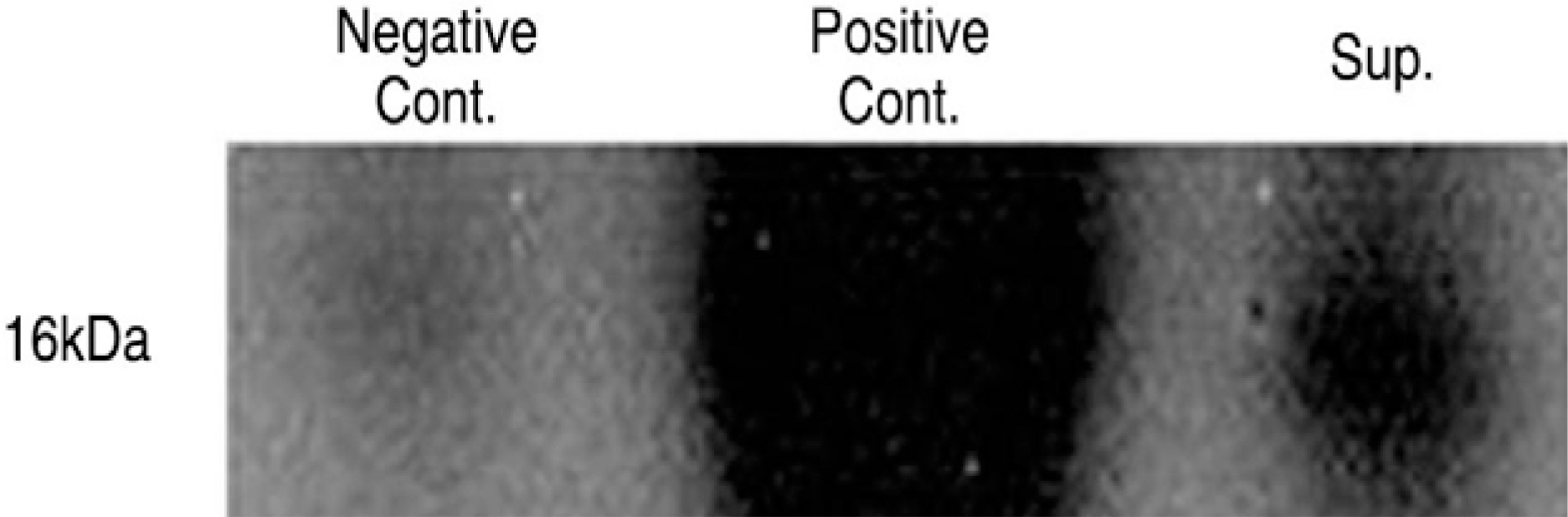
LF surgical specimens were obtained from patients with a lumbar spine stenosis, and the cells were isolated by enzymatic digestion. Each of the synovium tissues were weighed and recorded. Each tissue was cut into small pieces with a pair of scissors and then washed 3 times with PBS. The washed tissue pieces were then cultured for 96 hr at 37degrees C, 5% CO2 in DMEM/F-12-0.1% FBS with a density of 200 mg/ml medium. The supernatant was collected after 96 hr. In order to measure quantitatively the proliferation of cells, the AlamarBlue assay was used. The total cellular RNA was extracted from the cells and amplification reactions specific to the following types of cDNA were performed: the osteogenic master transcription factors, Dlx5, Runx2, osterix, and types collagen and osteocalcin. Alkaline phosphatase staining for the biochemical assay and western blotting for osteocalcin protein expression were performed.
RESULTS
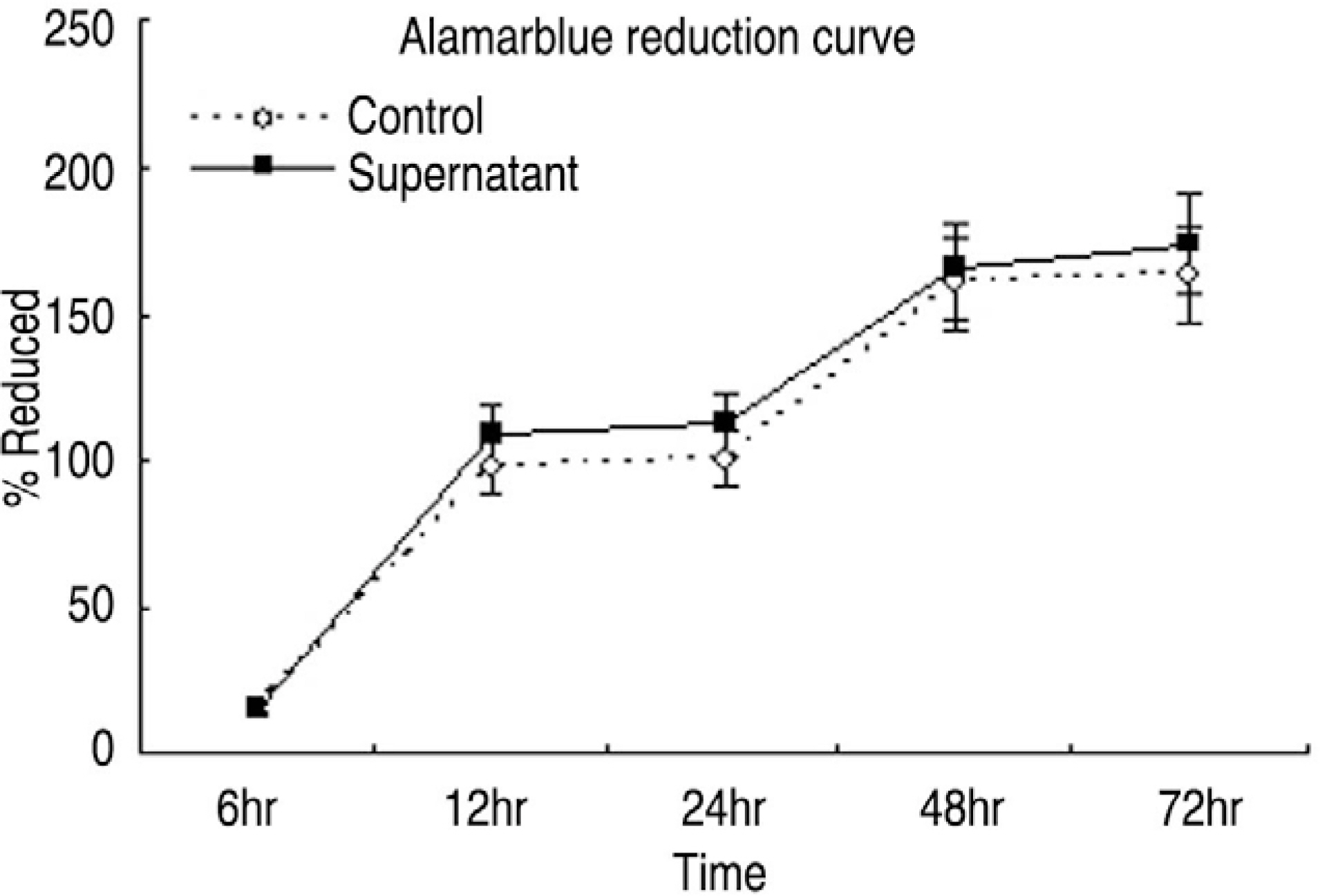
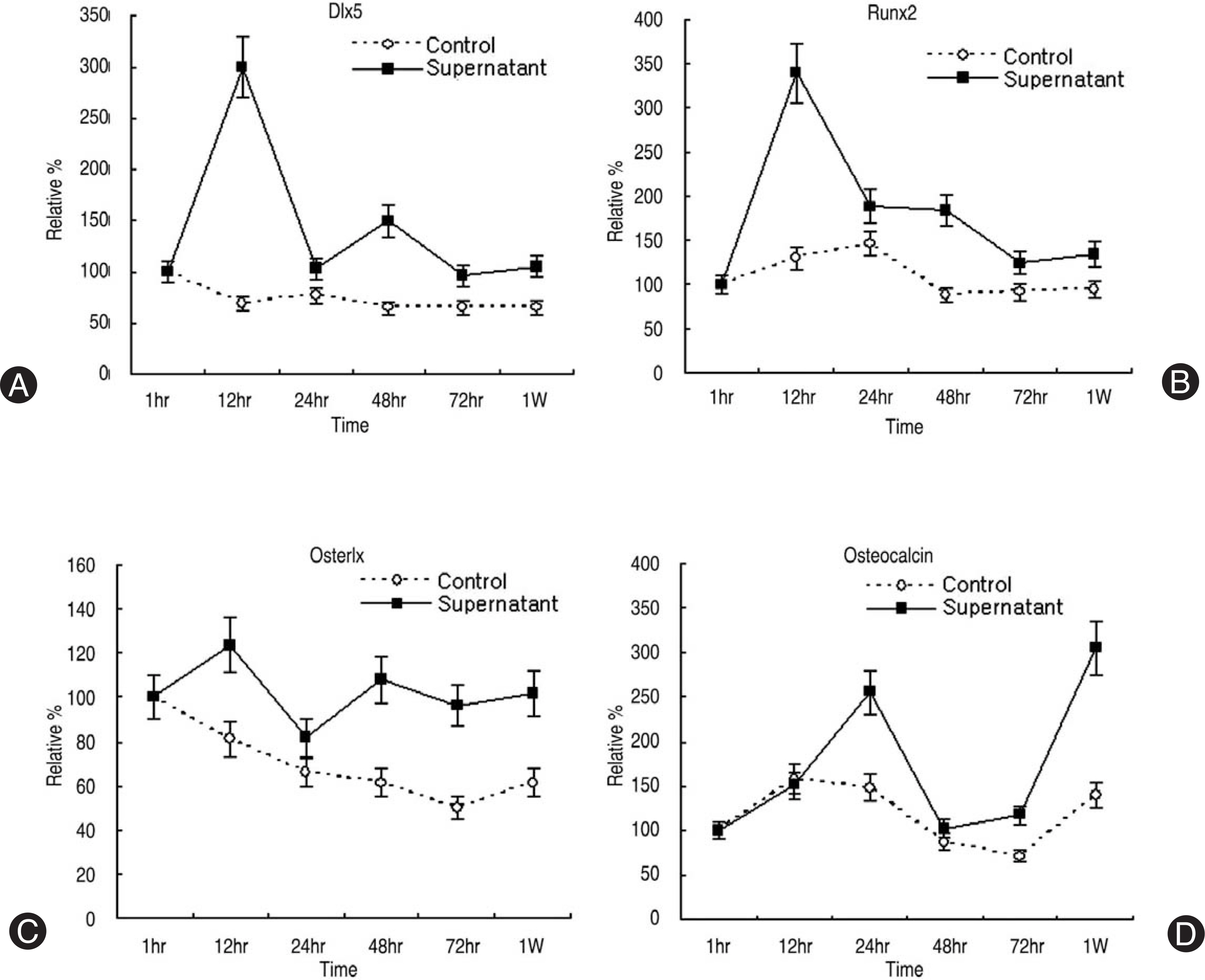
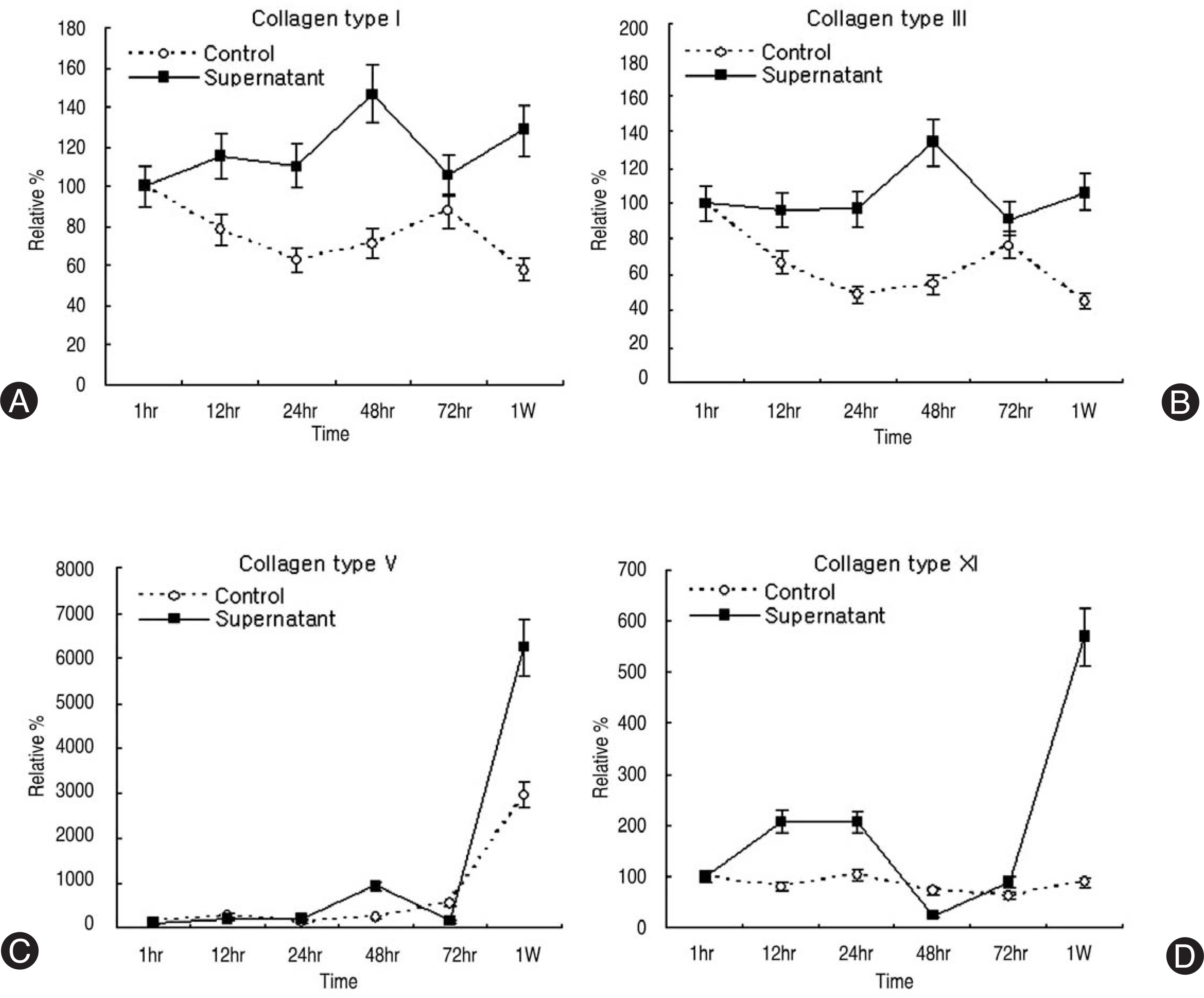
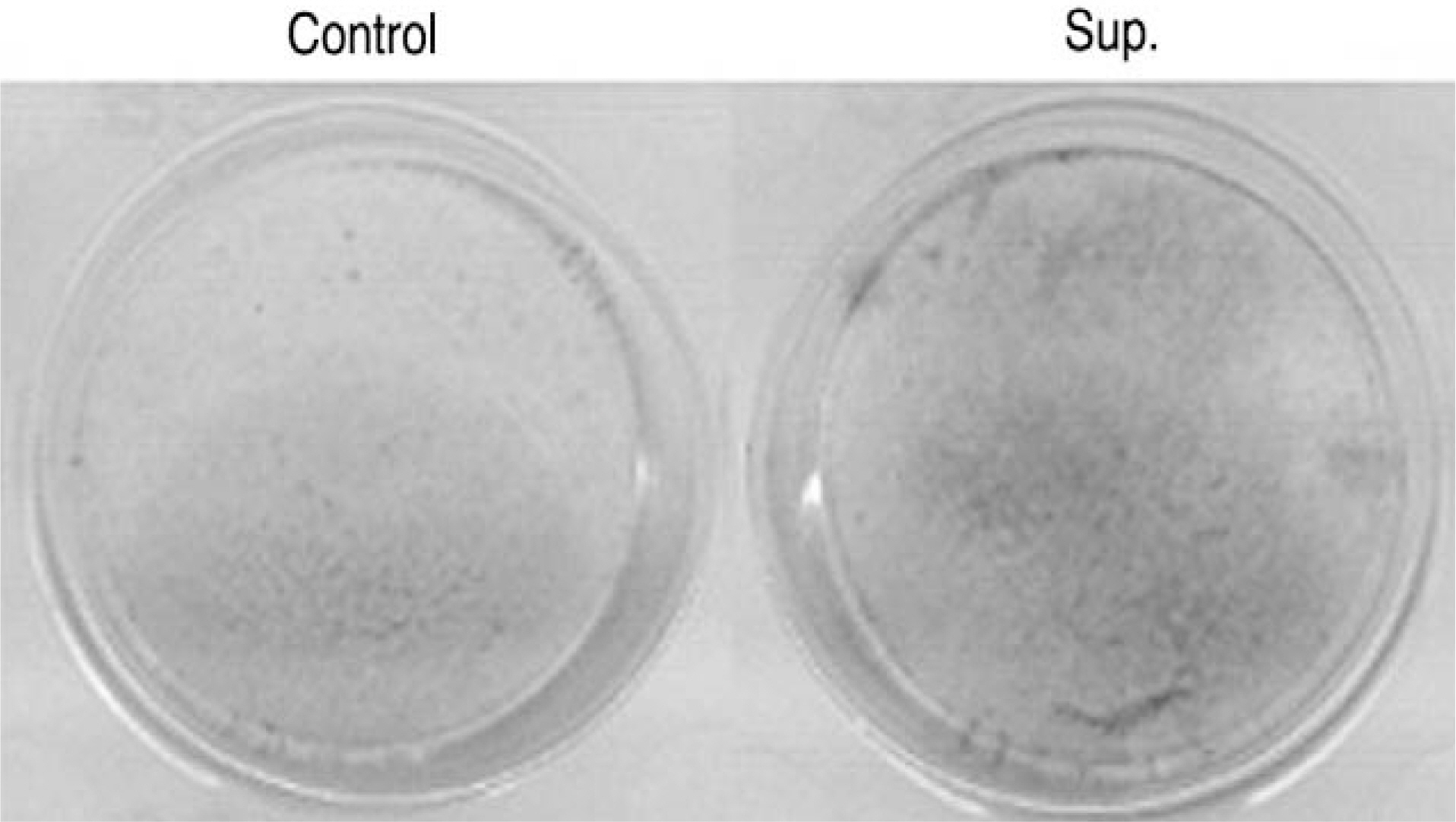
Human LF cells cultured with the supernatant from the facet synovium showed a slightly stronger AlamarBlue staining than the intensity of the control culture. RT-PCR revealed the upregulation of the osteogenic master transcription factors, Dlx5, Runx2, and osterix in the synovium supernatant group from one hour to 72 hours, and an increase in osteocalcin, types collagen I, III, V, XI levels from one hour to one week. LF cells cultured with the supernatant from the facet synovium showed positive staining for alkaline phosphatase. The level of the osteocalcin protein in the LF cells cultured with the supernatant from the facet synovium was higher than the control group.
Conclusions
The supernatant of the facet joint from patients with degenerative spinal stenosis affects LF cells by increasing the level of cellular proliferation, upregulating the mRNA expression of osteocalcin, types of collagen, osteogenic transcription factors, positive alkaline phosphatase staining, and osteocalcin protein expression. Therefore, degenerated synovial fluid from the facet joint is an important mechanism of LF hypertrophy and ossification.
MeSH Terms
-
Aging
Alkaline Phosphatase
Blotting, Western
Cell Proliferation
Cell Survival
Collagen
Constriction, Pathologic
Cytokines
Digestion
DNA, Complementary
Humans
Hyaline Cartilage
Hypertrophy*
Joints
Ligamentum Flavum*
Osteoarthritis
Osteocalcin
Osteogenesis
Phenotype
RNA
RNA, Messenger
Spinal Canal
Spinal Stenosis
Spine
Synovial Fluid*
Synovial Membrane
Transcription Factors
Up-Regulation
Zygapophyseal Joint
Alkaline Phosphatase
Collagen
Cytokines
DNA, Complementary
Osteocalcin
RNA
RNA, Messenger
Transcription Factors
Figure
Reference
-
1). Lee HM. Pathophysiology of lumbar spinal stenosis. J Kor Spine Surg. 2000; 7:100–105.2). Igarashi A, Kikuchi S, Konno S, Olmarker K. Inflammatory cytokines released from the facet joint tissue in degenerative lumbar spinal disorders. Spine. 2004; 29:2091–2095.
Article3). Keiseki K, Segami N, Sun W, Sato J, Fujimura K. Analysis of tumor necrosis factor-alpha, interleukin-6, interleukin-1beta, soluble tumor necrosis factor receptors I and II, interleukin-6 soluble receptor, interleukin-1 soluble receptor type II, interleukin-1 receptor antagonist, and protein in the synovial fluid of patients with temporo-mandibular joint disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005; 99:276–284.4). Lohmander LS, Atley LM, Pietka TA, Eyre DR. The release of crosslinked peptides from type II collagen into human synovial fluid is increased soon after joint injury and in osteoarthritise. Arthritis Rheum. 2003; 48:3130–3139.5). Lettesjo H, Nordstrom E, Strom H, et al. Synovial fluid cytokines in patients with rheumatoid arthritis or other arthritic lesions. Scand J Immunol. 1998; 48:286–292.6). Haynes MK, Hume EL, Smith JB. Phenotypic characterization of inflammatory cells from osteoarthritic synovium and synovial fluids. Clin Immunol. 2002; 105:315–325.
Article7). Specchia N, Pagnotta A, Gigante A, Logroscino G, Toesca A. Characterization of cultured human ligamentum flavum cells in lumbar spine stenosis. J Orthop Res. 2001; 19:294–300.
Article8). Okuda T, Baba I, Fujimoto Y, et al. The pathology of ligamentum flavum in degenerative lumbar disease. Spine. 2004; 29:1689–1697.
Article9). Li H, Zou Z, Baatrup A, Lind M, Bunger C. Cytokine profiles in conditioned media from culture human intervertebral disc tissue. Acta Orthop. 2005; 76:115–121.10). Nociari MM, Shalev A, Benias P, Russo C. A novel one-step, highly sensitive fluorometric assay to evaluate cell-mediated cytotoxicity. J Immunol Methods. 1998; 213:157–167.
Article11). Derfoul A, Carlberg AL, Tuan RS, Hall DJ. Differen-tial regulation of osteogenic marker gene expression by Wnt-3a in embryonic mesenchymal multipotential progen-itor cells. Differentiation. 2004; 72:209–223.
Article12). Luppen CA, Leclerc N, Noh T, et al. Brief bone mor-phogenetic protein 2 treatment of glucocorticoid-inhibited MC3T3-E1 osteoblasts rescues commitment-associated cell cycle and mineralization without alteration of Runx2. J Biol Chem. 2003; 278:44995–45003.
Article13). Ryoo HM, Hoffmann HM, Beumer T, et al. Stage-spe-cific expression of Dlx-5 during osteoblast differentiation: involvement in regulation of osteocalcin gene expression. Mol Endocrinol. 1997; 11:1681–1694.
Article14). Lee MH, Kwon TG, Park HS, John MW, Ryoo HM. BMP-2-induced osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem Biophys Res Com-mun. 2003; 309:689–694.
Article15). He J, Jiang J, Safavi KE, Spangberg LS, Zhu Q. Emdo-gain promotes osteoblast proliferation and proliferation and differentiation and stimulates osteoprotegerin expression. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 97:239–245.16). Nakatani T, Marui T, Hitora T, Doita M, Nishida K, Kurosaka M. Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factor-beta1. J Orthop Res. 2002; 20:1380–1386.17). Sherwood OD. Relaxin's physiological roles and other diverse actions. Endocrine Rev. 2004; 25:205–234.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Thoracic Myelopathy Due to Ossification of Ligamentum Flavum: Three cases Report
- Expression of Estrogen Receptor on Ligamentum Flavum and Facet Joint Capsule in Degenerative Spinal Stenosis
- As a Cause of Myelopathy in the Lower Thracic Spines ): Two Cases Report
- Focal Ligamentum Flavum Hypertrophy with Ochronotic Deposits: An Unusual Cause for Neurogenic Claudication in Alkaptonuria
- Myelopathy due to Ossification of Ligamentum Flavum