J Korean Diabetes Assoc.
2007 May;31(3):243-252. 10.4093/jkda.2007.31.3.243.
The Differences of Circulating Adiponectin Levels and Multimerization According to Obesity in Type 2 Diabetes Mellitus of Men
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Korea.
- 2College of Nursing, The Catholic University of Korea, Korea.
- KMID: 1523013
- DOI: http://doi.org/10.4093/jkda.2007.31.3.243
Abstract
-
BACKGROUND: Adiponectin is adipose tissue derived hormone, which has been shown to play an important role in the regulation of glucose and lipid metabolism. Low adiponectin levels are associated with obesity and diabetes and coronary artery disease. In addition to adiponectin level, the adiponectin multimerization and its ratio to total adiponectin have also affect on metabolic risk factors and insulin resistance. However, the adiponectin multimerization pattern in type 2 diabetes of Korean has not been established. We investigated adiponectin levels and adiponectin multimerization pattern according to obesity in type 2 diabetes males of Korean.
METHOD: The subjects of this study were 86 of diabetes patients and 89 of control subjects whose fasting blood glucose was below 110 mg/dL. They were divided into two subgroup, non-obese and obese, according to BMI (non-obese 25 < BMI). Anthropometric parameter and other metabolic risk factors were measured. Insulin resistance was presented by HOMA-IR. Plasma adiponectin level was measured by radioimmunoassay method. Adiponectin multimerization was fractionated by SDS-PAGE under non-reducing and non-heat denaturing state and performed immunoblotting.
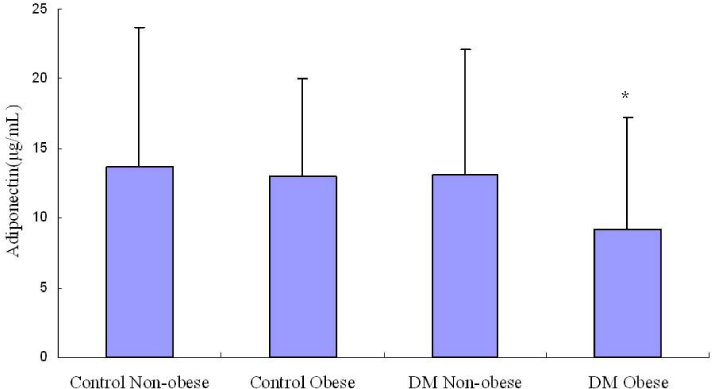
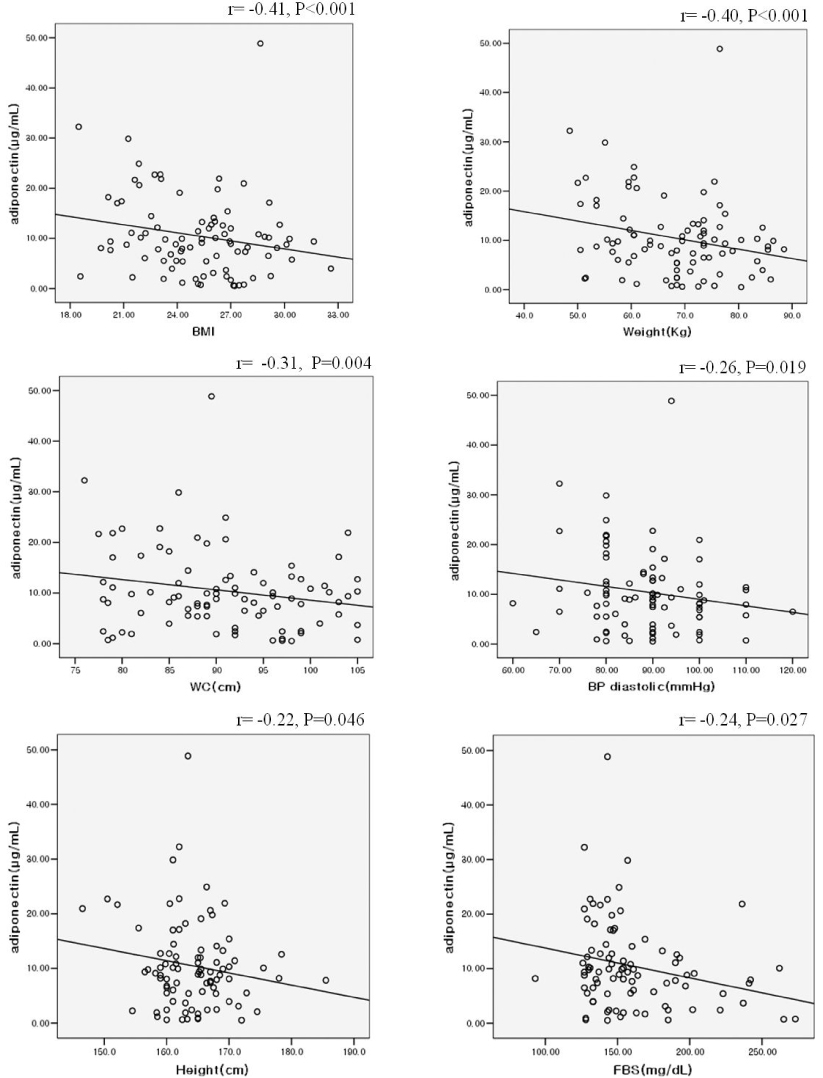
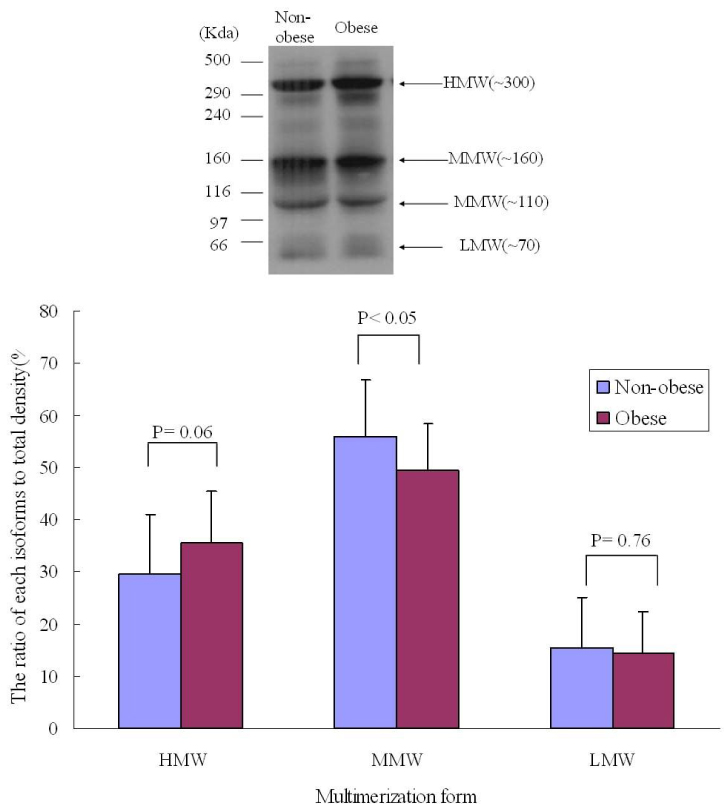
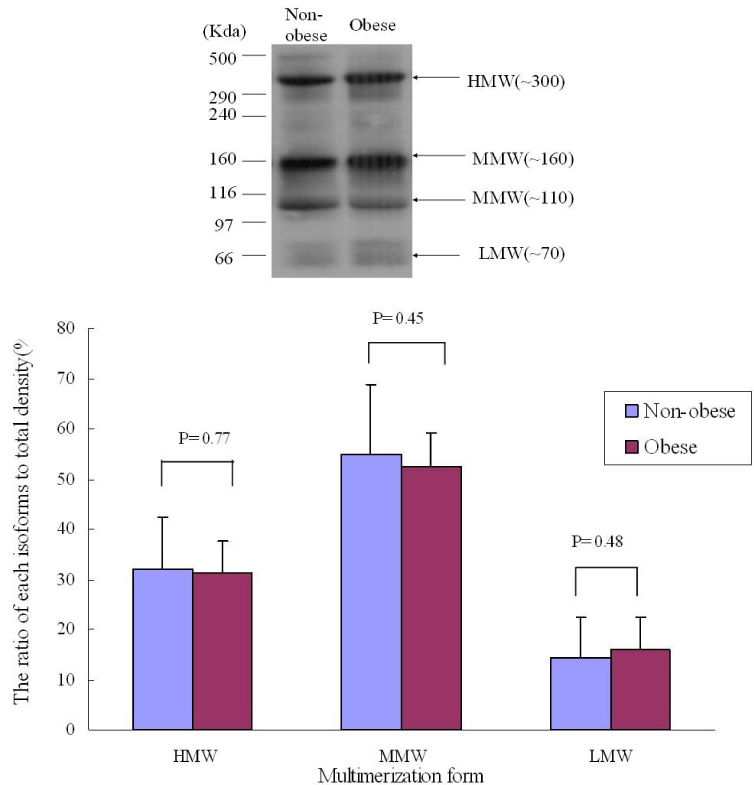
RESULT: Serum adiponectin levels were significantly reduced in obese than non obese group in diabetes patients (7.73 +/- 5.2 versus 12.56 +/- 8 microgram/mL, P = 0.003). Correlational analyses demonstrated that BMI, body weight, waist circumference, diastolic pressure, glucose and height correlated significantly with adiponectin levels in the diabetes patients. The HOMA-IR did not affect the plasma adiponectin levels in diabetic patients. There were no differences in adiponectin multimerization distribution and ratio between obese and non-obese group in the diabetes, however middle molecular weight multimers (MMW, ~110~160 Kda, hexamer) ratio in the control subjects were significantly reduced in obese group than non-obese group (49 +/- 9 versus 56 +/- 11%, P < 0.05).
CONCLUSION
The adipoenctin levels were lower in obese than non-obese group of diabetes males in Korea. Aiponectin levels correlated with BMI and weight but not insulin resistance. The differences of adiponectin multimerization distribution and ratio between obese and non-obese group in diabetes were not detected.
MeSH Terms
-
Adiponectin*
Adipose Tissue
Blood Glucose
Blood Pressure
Body Weight
Coronary Artery Disease
Diabetes Mellitus, Type 2*
Electrophoresis, Polyacrylamide Gel
Fasting
Glucose
Humans
Immunoblotting
Insulin Resistance
Korea
Lipid Metabolism
Male
Molecular Weight
Obesity*
Plasma
Radioimmunoassay
Risk Factors
Waist Circumference
Adiponectin
Blood Glucose
Glucose
Figure
Reference
-
1. Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001. 86:1930–1935.2. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin in obesity. Biochem Biophys Res Commun. 1999. 257:79–83.3. Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CI, Tai TY, Chung LM. Weight reduction increases plasma levels of adipocyte-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metabo. 2001. 96:3815–3819.4. Abbasi F, Chu JW, Lamendola C, Mclaughlin T, Hayden J, Reaven GM, Reaven PD. Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes. 2004. 53:585–590.5. Waki H, Yamauchi T, Kamon J, Ito Y, Uckida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. J Biol Chem. 2003. 278:40352–40363.6. Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzshneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004. 279:12152–12162.7. Taso TS, Thomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, Heuse JE, Lodish HF. Role of disulfide bonds in ACrp 30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem. 2003. 278:50810–50817.8. Chang SA, Kim HS, Yoon KH, Ko SH, Kwon HS, Kim SR, Lee WC, Yoo SJ, Son HS, Cha BY, Lee KW, Son HY, Kang SK. Body mass index is the most important determining factor for the degree of insulin resistance in non-obese type 2 diabetic patients in Korea. Metabolism. 2004. 53:142–146.10. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985. 28:412–419.11. Kim MJ, Yoo KH, Park HS, Chung SM, Jin CJ, Lee Y, Shin YG, Chung CH. Plasma adiponectin and insulin resistance in Korean type 2 Diabetes mellitus. Yonsei Med J. 2005. 46:42–50.13. Ferris WF, Naran NH, Crowther NJ, Rheeder P, Merwe LV, Chetty N. The relationship between insulin sensitivity and serum adiponectin levels in three population groups. Norm Metab Res. 2005. 37:695–701.14. Hulver MA, Saleh O, MacDonald KG, Pories WJ, Barakat HA. Ethnic differences in adiponectin levels. Metabolism. 2004. 53:1–3.15. Zietz B, Herfarth H, Paul G, Ehling A, Muller-Ladner U, Scholmerich J, Schaffler A. Adiponectin represents an independent cardiovascular risk factor predicting serum HDL-cholesterol levels in type 2 diabetes. FEBS Lett. 2003. 454:103–104.16. Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002. 87:2764–2769.17. Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, Mantzoros CS. Serum adiponectin levels are inversely associated with central fat distribution and estrogen levels but are not directly regulated by acute fasting and leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab. 2003. 88:4823–4831.18. Huang KC, Chen CL, Chung LM, Ho SR, Tai TY, Yang WS. Plasma adiponectin levels and blood pressures in nondiabetic adolescents females. J Clin Endocrinol Metab. 2003. 42:76–81.19. Furuhashi M, Ura N, Higasshiura K, Murakami H, Tanaka M, Moniwa N, Yoshida D, Shimamoto K. Blokcade renin-angiotensin system increases adiponectin concentration in patients with essential hypertension. Hypertension. 2003. 42:76–81.20. Adamczak M, Wiecek A, Funahashi T, Chudek J, Kokot F, Matsuzawa Y. Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2003. 16:72–75.21. Kazumi T, Kawaguchi A, Sakai K, Hirano T, Yoshino G. Young men with high-normal blood pressure have lower serum adiponectin, smaller LDL size, and higher elevated heart rate than those with optimal blood pressure. Diabetes Care. 2002. 25:971–976.22. Mallamaci F, Zocaali C, Cuzzola F, Tripepi G, Cutrupi S, Parlongo S, Tanaka S, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y. Adiponectin in essential hypertension. J Nephrol. 2002. 15:507–511.23. Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003. 46:459–469.24. Tonelli J, Li W, Kishore P, Pajvani UB, Kwon E, Weaver C, Scherer PE, Hawkins M. Mechanism of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes. 2004. 53:1621–1629. [erratum in Diabetes 54:587, 2005].25. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki S, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty acid-oxidation by activating AMP-activated protein kinase. Nat Med. 2002. 8:1288–1295.26. Lara-Castro C, Luo L, Wallace P, Klein R, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006. 55:249–259.27. Xu A, Chan KW, Hoo RLC, Wang Y, Tan KCB, Zhang J, Chen B, Lam MC, Tse C, Cooper GJS, Lam KSL. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005. 280:18073–18080.28. Bobbert T, Rochlitz H, Wegewitz U, Akpulat S, Mai K, Weickert M, Mohlig M, Pfeiffer A, Spranger J. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 2005. 54:2712–2719.29. Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, Matsuda M, Kondo H, Furuyama N, Kihara S, Nakamura T, Tochino Y, Funahashi T, Matsuzawa Y. Androgen decrease plasma adiponectin, and insulin-sensitizing adipocyte-derived protein. Diabetes. 2002. 51:2734–2741.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Plasma Adiponectin and Polymorphism of Adiponectin Gene in the Development of Type 2 Diabetes Mellitus
- The Association Between Adiponectin and Diabetes in the Korean Population
- Adiponectin and Diabetes Mellitus
- Impact of the Serum Adiponectin Concentration on the Progression of Carotid Atherosclerosis in Patient with Type 2 Diabetes Mellitus
- The Plasma Adiponectin Levels in Patients with Newly Diagnosed Type 2 Diabetes