Immune Netw.
2009 Apr;9(2):64-73. 10.4110/in.2009.9.2.64.
15-Deoxy-Delta(12,14)-Prostaglandin J2 Upregulates the Expression of LPS-Induced IL-8/CXCL8 mRNA in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats
- Affiliations
-
- 1Department of Microbiology, College of Medicine, and Aging-associated Vascular Disease Research Center, Yeungnam University, Daegu, Korea. heesun@med.yu.ac.kr
- KMID: 1474597
- DOI: http://doi.org/10.4110/in.2009.9.2.64
Abstract
- BACKGROUND
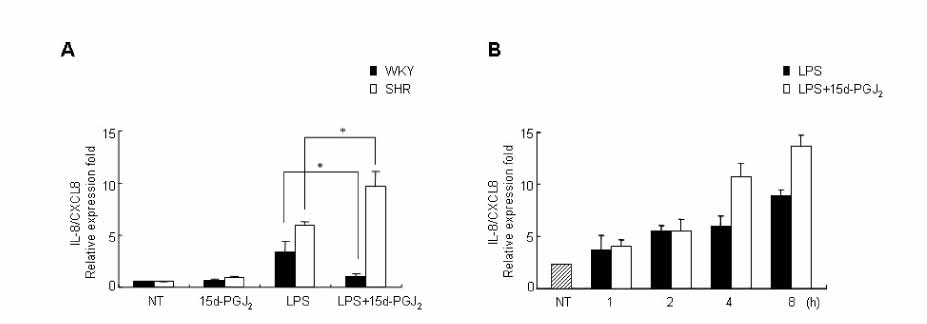
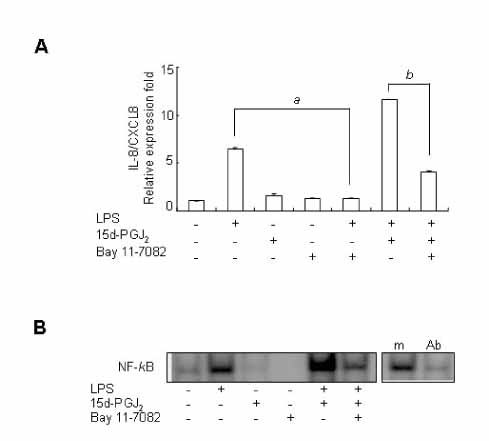
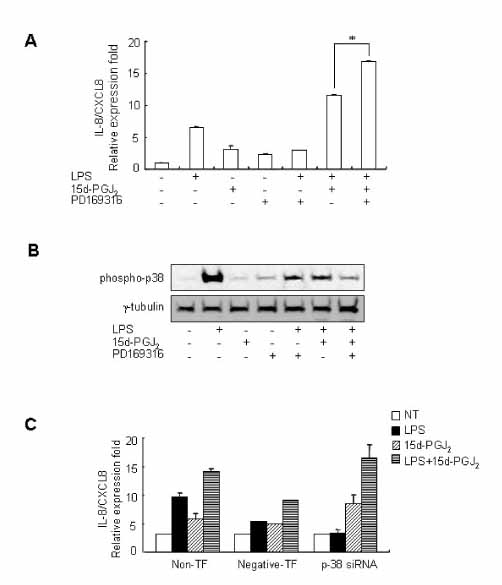
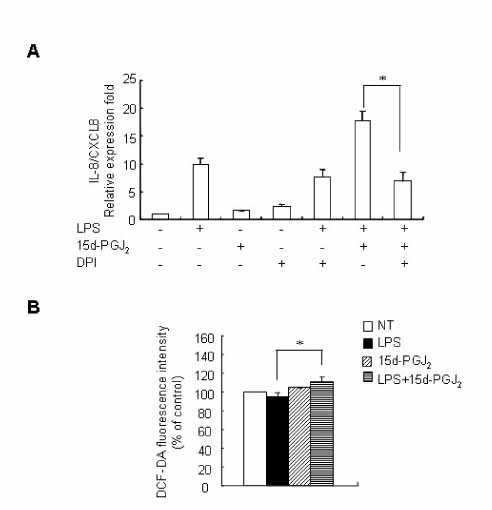
15d-PGJ2 has been known to act as an anti-inflammatory agent and has anti-hypertensive effects. As a result of these properties, we examined the effect of 15d-PGJ2 on the LPS-induced IL-8/CXCL8 mRNA expression in VSMCs from SHR. METHODS: Effect and action mechanism of 15d-PGJ2 on the expression of LPS-induced IL-8/CXCL8 mRNA in VSMCs from SHR and WKY were examined by using real-time polymerase chain reaction, electrophoretic mobility shift assay for NF-kappaB avtivity, Western blotting analysis for ERK and p38 phosphorylation and flow cytometry for NAD(P)H oxidase activity. RESULTS: 15d-PGJ2 decreased the expression of LPS-induced IL-8/CXCL8 mRNA in WKY VSMCs, but increased the expression of LPS-induced IL-8/CXCL8 mRNA in SHR VSMCs. The upregulatory effect of 15d-PGJ2 in SHR VSMCs was mediated through PPAR gamma, and dependent on NF-kappaB activation and ERK phosphorylation. However, inhibition of the p38 signaling pathway augmented the upregulatory effect of 15d-PGJ2 on LPS-induced IL-8/CXCL8 mRNA. A NAD(P)H oxidase inhibitor inhibited the upregulatory effect of 15d-PGJ2 on LPS-induced IL-8/CXCL8 mRNA expression in SHR VSMCs, and an increase in NAD(P)H oxidase activity was detected in SHR VSMCs treated with 15d-PGJ2/LPS. CONCLUSION: Our results indicate that the upregulatory effect of 15d-PGJ2 on LPS-induced IL-8/CXCL8 expression in SHR VSMCs is mediated through the PPAR gamma and ERK pathway, and may be related to NAD(P)H oxidase activity. However, p38 inactivation may also play an important role in 15d-PGJ2/LPS-induced IL-8/CXCL8 expression in SHR VSMCs.
Keyword
MeSH Terms
-
Blotting, Western
Electrophoretic Mobility Shift Assay
Flow Cytometry
MAP Kinase Signaling System
Muscle, Smooth, Vascular
NADPH Oxidase
NF-kappa B
Phosphorylation
PPAR gamma
Prostaglandin D2
Rats, Inbred SHR
Real-Time Polymerase Chain Reaction
RNA, Messenger
NADPH Oxidase
NF-kappa B
PPAR gamma
Prostaglandin D2
RNA, Messenger
Figure
Reference
-
1. Alexander RW. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995. 25:155–161.
Article2. Capers Q 4th, Alexander RW, Lou P, De Leon H, Wilcox JN, Ishizaka N, Howard AB, Taylor WR. Monocyte chemoattractant protein-1 expression in aortic tissues of hypertensive rats. Hypertension. 1997. 30:1397–1402.
Article3. Rodríguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol. 2004. 286:F606–F616.
Article4. Zhang Y, Griendling KK, Dikalova A, Owens GK, Talyor WR. Vascular hypertrophy in angiotensin II-induced hypertension is mediated by vascular smooth muscle cell-derived H2O2. Hypertension. 2005. 46:732–737.
Article5. Gerszten RE. Pleiotropic effects of chemokines in vascular lesion development. Arterioscler Thromb Vasc Biol. 2002. 22:528–529.
Article6. Luster AD. Chemokines - chemotactic cytokines that mediate inflammation. N Engl J Med. 1998. 338:436–445.
Article7. Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999. 398:718–723.
Article8. Boekholdt S, Peters R, Hack CE, Day NE, Luben R, Bingham SA, Wareham NJ, Reitsma PH, Khaw K. IL-8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women. the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2004. 24:1503–1508.
Article9. Buemi M, Marino D, Floccari F, Ruello A, Nosto L, Aloisi C, Marino MT, Di Pasquale G, Corica F, Frisina M. Effect of interleukin 8 and ICAM-1 on calcium-dependent outflow of K+ in erythrocytes from subjects with essential hypertension. Curr Med Res Opin. 2004. 20:19–24.
Article10. Kim HY, Kang YJ, Song IH, Choi HC, Kim HS. Upregulation of Interleukin-8/CXCL8 in vascular smooth muscle cells from spontaneously hypertensive rats. Hypertens Res. 2008. 31:515–523.
Article11. Dobrian AD, Schriver SD, Khraibi AA, Prewitt RL. Pioglitazone prevents hypertension and reduces oxidative stress in diet-induced obesity. Hypertension. 2004. 43:48–56.
Article12. Fukuda N, Hu WY, Teng J, Chikara S, Nakayama K, Kanmatsuse K. Troglitazone inhibits growth and improves insulin signaling by suppression of angiotensin II action in vascular smooth muscle cells from spontaneously hypertensive rats. Atherosclerosis. 2002. 163:229–239.
Article13. Wakino S, Hayashi K, Tatematsu S, Hasegawa K, Takamatsu I, Kanda T, Homma K, Yoshioka K, Sugano N, Saruta T. Pioglitazone lowers systemic asymmetric dimethylarginine by inducing dimethylarginine dimethylaminohydrolase in rats. Hypertens Res. 2005. 28:255–262.
Article14. Calnek DS, Mazzella L, Roser S, Roman J, Hart CM. Peroxisome proliferatorsactivated receptor gamma ligands increase release of nitric oxide from endothelial cells. Arterioscler Thromb Vasc Biol. 2003. 23:52–57.
Article15. Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998. 391:82–86.
Article16. Chastine Bell-Parikh L, Ide T, Lawson JA, Mcncmara P, Reilly M, Fitzgerald GA. Biosynthesis of 15-deoxy-delta 12,14- PGJ2 and the ligation of PPARgamma. J Clin Invest. 2003. 112:945–955.17. Benkirane K, Amiri F, Diep QN, Mabrouk ME, Schiffrin EL. PPAR-gamma inhibits ANG II-induced cell growth via SHIP2 and 4E-BP1. Am J Physiol Heart Circ Physiol. 2006. 290:H390–H397.18. Law RE, Goetze S, Xi XP, Jackson S, Kawano Y, Demer L, Fishbein MC, Meehan WP, Hsueh WA. Expression and function of PPARgamma in rat and human vascular smooth muscle cells. Circulation. 2000. 101:1311–1318.
Article19. Lim HJ, Lee KS, Lee S, Park JH, Choi HE, Go SH, Kwak HJ, Park HY. 15d-PGJ2 stimulates HO-1 expression through p38 MAP kinase and Nrf-2 pathway in rat vascular smooth muscle cells. Toxicol and Appl Pharmacol. 2007. 223:20–27.
Article20. Cuzzocrea S, Wayman NS, Mazzon E, Dugo L, Paola R, Serraino I, Britti D, Chatterjee PK, Caputi AP, Thiemermann C. The cyclopentenone prostaglandin 15-deoxy-Delta(12,14)-prostaglandin J(2) attenuates the development of acute and chronic inflammation. Mol Pharmacol. 2002. 61:997–1007.
Article21. Reilly CM, Oates JC, Suidan J, Crosby MB, Halushka PV, Gilkeson GS. Prostaglandin J2 inhibition of mesangial cell iNOS expression. Clin Immunol. 2001. 3:337–345.22. Sawano H, Haneda M, Sugimoto T, Inoki K, Koya D, Kikkawa R. 15-Deoxy-Delta12,14-prostaglandin J2 inhibits IL-1beta-induced cyclooxygenase-2 expression in mesangial cells. Kidney Int. 2002. 61:1957–1967.
Article23. Fu Y, Luo N, Lopes-Virella MF. Upregulation of interleukin-8 expression by prostaglandin J2 (15d-PGJ2) in human THP-1 macrophages. Atherosclerosis. 2002. 160:11–20.
Article24. Harris SG, Smith RS, Phipps RP. 15-deoxy-Delta 12,14-PGJ2 induces IL-8 production in human T cells by a mitogen-activated protein kinase pathway. J Immunol. 2002. 168:1372–1379.
Article25. Kim HY, Kim HK, Kim JR, Kim HS. Upregulation of LPS-induced chemokine KC expression by 15-deoxy-delta12,14-prostaglandin J2 in mouse peritoneal macrophages. Immunol Cell Biol. 2005. 83:286–293.
Article26. Kim HY, Kim HS. Upregulation of MIP-2 (CXCL2) expression by 15-deoxy-Delta(12,14)-prostaglandin J(2) in mouse peritoneal macrophages. Immunol Cell Biol. 2007. 85:60–67.
Article27. Kelly G, Robert B, Chris R, Gary G, Perry H, James C. Differential effects of 15-deoxy-Delta(12,14)-PGJ2 and a peroxisome proliferators-activated receptor gamma agonist on macrophage activation. J Leukoc Biol. 2001. 69:631–663.28. Ricote M, Huang JT, Welch JS, Glass CK. The peroxisome proliferator-activated receptor gamma (PPARgamma) as a regulator of monocyte/macrophage function. J Leukoc Biol. 1999. 66:733–739.
Article29. Zhang X, Wang JM, Gong WH, Mukaida N, Young HA. Differential regulation of chemokine gene expression by 15-deoxy-delta 12,14 prostaglandin J2. J Immunol. 2001. 166:7104–7111.
Article30. Wakino S, Hayashi H, Kanda T, Tatematsu S, Homma K, Yoshioka K, Taksmatsu I, Saruta T. Peroxisome proliferatior-activated receptor gamma ligands inhibit Rho/Rho kinase pathway by inducing protein tyrosine phosphatase SHP-2. Circ Res. 2004. 95:e45–e55.31. Guyton K, Bond R, Reilly C, Gileson G, Halushka P, Cook J. Differential effects of 15-deoxy-delta(12,14)-prostaglandin J2 and a peroxisome proliferator-activatedreceptor gamma agonist on macrophage activation. J Leukoc Biol. 2001. 69:631–638.32. Kim HY, Kim JR, Kim HS. Upregulation of lipopolysaccharide-induced interleukin-10 by prostaglandin A1 in mouse peritoneal macrophages. J Microbiol Biotechnol. 2008. 18:1170–1178.33. Bureau F, Desmet C, Melotte D, Jaspar F, Volanti C, Vanderplasschen A, Pastoret PP, Piette J, Lekeux P. A proinflammatory role for the cyclopentenone prostaglandins at low micromolar concentrations. oxidative stress-induced extracellular signal-regulated kinase activation without NF-kappa B inhibition. J Immunol. 2002. 168:5318–5325.
Article34. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998. 391:79–82.
Article35. Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Galss CK. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A. 2000. 97:4844–4849.
Article36. Wilmer WA, Dixon C, Lu L, Hilbelink T, Rovin BH. A cyclopentenone prostaglandin activates mesangial MAP kinase independently of PPARgamma. Biochem Biophys Res Comm. 2001. 281:57–62.
Article37. Takeda K, Ichiki T, Tokunou T, Iino N, Takeshita A. 15-Deoxy-delta 12,14-prostaglandin J2 and thiazolidinediones activate the MEK/ERK pathway through phosphatidylinositol 3-kinase in vascular smooth muscle cells. J Biol Chem. 2001. 276:48950–48955.
Article38. Jung YD, Fan F, McConkey DJ, Jean ME, Liu W, Reinmuth N, Stoeltzing O, Ahmad SA, Parikh AA, Mukaida N, Ellis LM. Role of p38 MAPK, AP-1 and NF-kappaB in interleukin-1beta-induced IL-8 expression in human vascular smooth muscle cells. Cytokine. 2002. 18:206–213.
Article39. Zhao ML, Brosnan CF, Lee SC. 15-deoxy-delta (12,14)-PGJ2 inhibits astrocyte IL-1 signaling. inhibition of NF-kappaB and MAP kinase pathways and suppression of cytokine and chemokine expression. J Neuroimmunol. 2004. 153:132–142.
Article40. Thannickal VJ, Fanburg BL. Reative oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000. 279:L1005–L1028.41. Cruzado MC, Risler NR, Miatello RM, Yao G, Schiffrin EL, Touyz RM. Vascular smooth muscle cell NAD(P)H oxidase activity during the development of hypertension. effect of angiotensin II and role of insulinkike growth factor-1 receptor transactivation. Am J Hypertens. 2005. 18:81–87.
Article42. Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999. 85:753–766.
Article43. Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity upstream mediators. Circ Res. 2002. 91:406–413.44. Touyz RM, Schiffrin EL. Ang II-stimulated generation of reactive oxygen species in Human vascular smooth muscle cells in mediated via PLD-dependent pathways. Hypertension. 1999. 34:976–982.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- IL-8/CXCL8 Upregulates 12-Lipoxygenase Expression in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats
- Expression of Endothelin-1 by Stimulation with CXCL8 in Mouse Peritoneal Macrophages
- 15-Deoxy-Δ12,14 -prostaglandin J2 Induces Epithelial-tomesenchymal Transition in Human Breast Cancer Cells and Promotes Fibroblast Activation
- Downregulation of Angiotensin II-Induced 12-Lipoxygenase Expression and Cell Proliferation in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats by CCL5
- Porphyromonas gingivalis Lipopolysaccharide Regulates Migration of Vascular Smooth Muscle Cells