Lab Anim Res.
2012 Jun;28(2):109-114. 10.5625/lar.2012.28.2.109.
Altered expression of gamma-secretase components in animal model of major depressive disorder induced by reserpine administration
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- KMID: 1436705
- DOI: http://doi.org/10.5625/lar.2012.28.2.109
Abstract
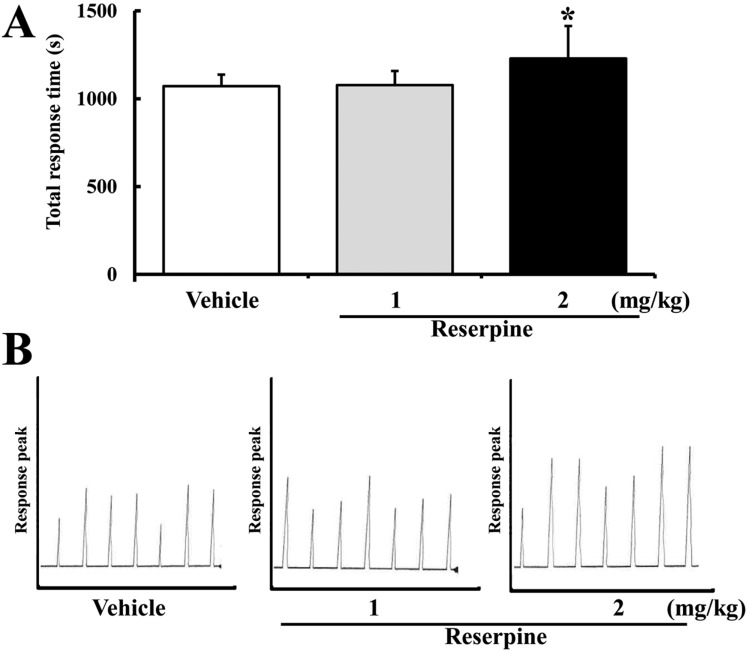
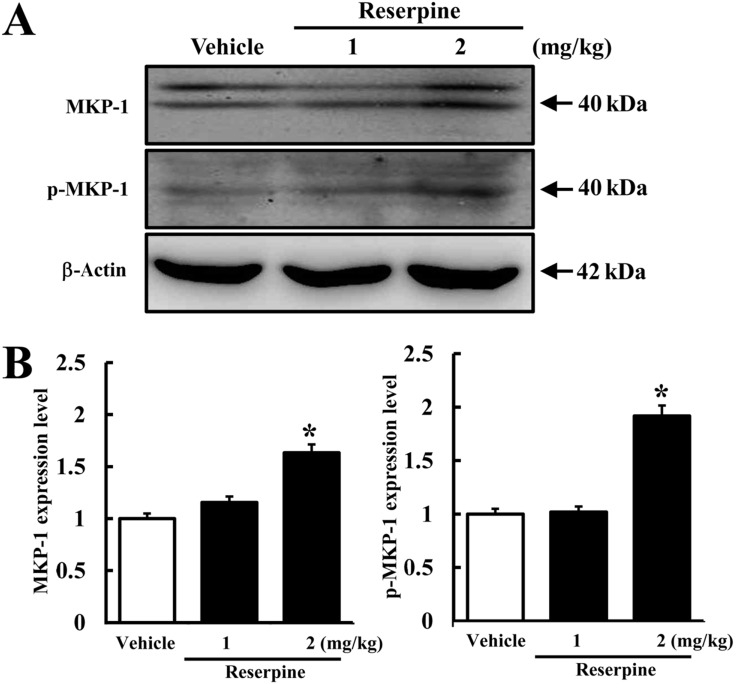
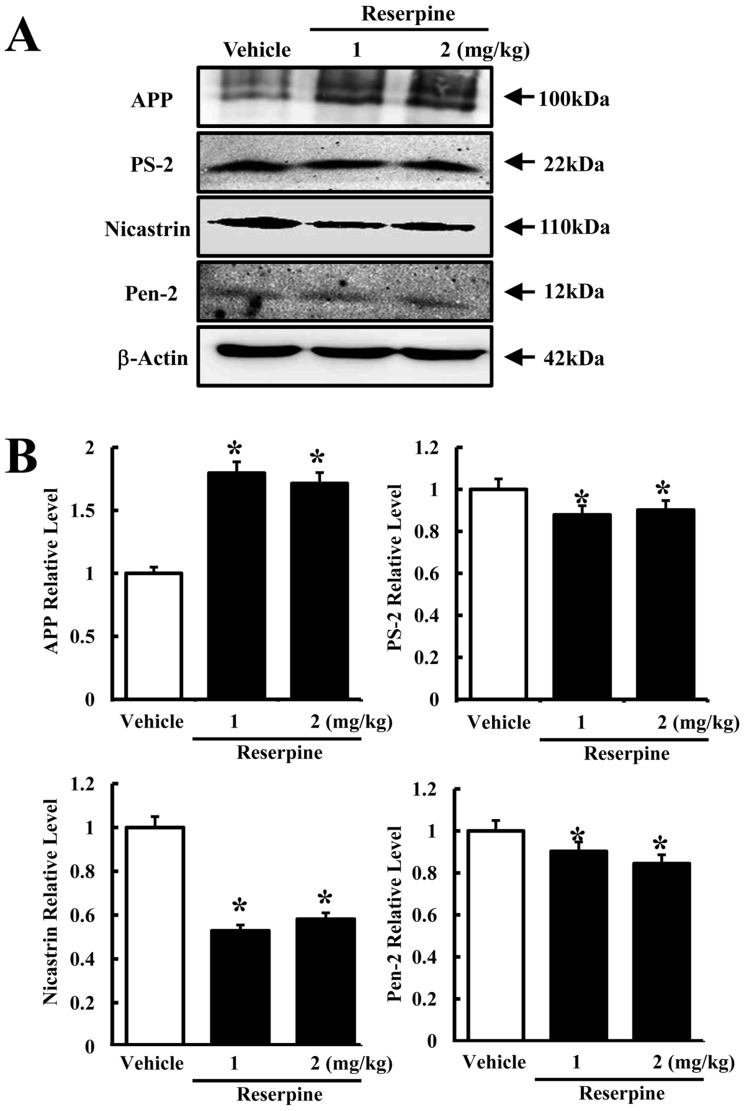
- Altered expression of neurotrophic factors as well as neuroinflammation is commonly associated with Major depressive disorder (MDD) and Alzheimer's disease (AD). To investigate whether or not reserpine-induced MDD affects the expression of AD-related proteins, the expression of gamma-secretase components and substrate were measured in brains of ICR mice following reserpine treatment for 15 days. In active avoidance test, total response time and peak slightly increased in the 2 mg/kg reserpine (RSP2)-treated group compared to vehicle-treated group (P<0.05). Expression and phosphorylation of MKP-1, which is a key factor in MDD pathology, were both higher in the RSP2-treated group than the vehicle- and 1 mg/kg reserpine (RSP1)-treated groups (P<0.02). Furthermore, full-length expression of amyloid precursor protein (APP) was enhanced in the RSP1 and RSP2-treated groups compared to the vehicle-treated group, whereas expression of gamma-secretase components decreased (P<0.03). Among the three components of the gamma-secretase complex, nicastrin protein underwent the largest decrease in expression, as detected by Western blotting (P<0.03). Therefore, the data presented here provide additional evidence about the pathological correlation between MDD and AD.
MeSH Terms
-
Alzheimer Disease
Amyloid
Amyloid Precursor Protein Secretases
Animals
Blotting, Western
Brain
Depressive Disorder, Major
Membrane Glycoproteins
Mice
Mice, Inbred ICR
Models, Animal
Nerve Growth Factors
Phosphorylation
Proteins
Reaction Time
Reserpine
Amyloid
Amyloid Precursor Protein Secretases
Membrane Glycoproteins
Nerve Growth Factors
Proteins
Reserpine
Figure
Reference
-
1. Sierksma AS, van den Hove DL, Steinbusch HW, Prickaerts J. Major depression, cognitive dysfunction and Alzheimer's disease: is there a link? Eur J Pharmacol. 2010; 626(1):72–82. PMID: 19837053.
Article2. Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001; 178:200–206. PMID: 11230029.
Article3. Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lonnqvist J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008; 106(1-2):1–27. PMID: 17707915.
Article4. Wuwongse S, Chang RC, Law AC. The putative neurodegenerative links between depression and Alzheimer's disease. Prog Neurobiol. 2010; 91(4):362–375. PMID: 20441786.
Article5. Haavik J, Blau N, Thony B. Mutations in human monoamine-related neurotransmitter pathway genes. Hum Mutat. 2008; 29(7):891–902. PMID: 18444257.
Article6. Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009; 164(1):300–330. PMID: 19358877.
Article7. Matrone C, Ciotti MT, Mercanti D, Marolda R, Calissano P. NGF and BDNF signaling control amyloidogenic route and Abeta production in hippocampal neurons. Proc Natl Acad Sci USA. 2008; 105(35):13139–13144. PMID: 18728191.8. Leonard BE, Myint A. Inflammation and depression: is there a causal connection with dementia? Neurotox Res. 2006; 10(2):149–160. PMID: 17062376.
Article9. Guerreiro RJ, Santana I, Brás JM, Santiago B, Paiva A, Oliveira C. Peripheral inflammatory cytokines as biomarkers in Alzheimer's disease and mild cognitive impairment. Neurodegener Dis. 2007; 4(6):406–412. PMID: 17934323.
Article10. Richartz-Salzburger E, Batra A, Stransky E, Laske C, Kohler N, Bartels M, Buchkremer G, Schott K. Altered lymphocyte distribution in Alzheimer's disease. J Psychiatr Res. 2007; 41(1-2):174–178. PMID: 16516234.11. Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol. 2009; 210(1-2):3–12. PMID: 19269040.12. Song C, Halbreich U, Han C, Leonard BE, Luo H. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry. 2009; 42(5):182–188. PMID: 19724980.
Article13. Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010; 15(5):501–511. PMID: 18825147.
Article14. Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS. A negative regulator of MAP kinase causes depressive behavior. Nat Med. 2010; 16(11):1328–1332. PMID: 20953200.
Article15. Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995; 269(5226):973–977. PMID: 7638622.
Article16. Van Broeckhoven C, Backhovens H, Cruts M, De Winter G, Bruyland M, Cras P, Martin JJ. Mapping of a gene predisposing to early-onset Alzheimer's disease to chromosome 14q24.3. Nat Genet. 1992; 2(4):335–339. PMID: 1303290.
Article17. Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001; 81(2):741–766. PMID: 11274343.
Article18. Williamson J, Goldman J, Marder KS. Genetic aspects of Alzheimer disease. Neurologist. 2009; 15(2):80–86. PMID: 19276785.
Article19. Arya U, Dwivedi H, Subramaniam JR. Reserpine ameliorates Abeta toxicity in the Alzheimer's disease model in Caenorhabditis elegans. Exp Gerontol. 2009; 44(6-7):462–466. PMID: 19264117.20. Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006; 313(5793):1604–1610. PMID: 16902091.
Article21. Steinkraus KA, Smith ED, Davis C, Carr D, Pendergrass WR, Sutphin GL, Kennedy BK, Kaeberlein M. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008; 7(3):394–404. PMID: 18331616.
Article22. Pakaski M, Bjelik A, Hugyecz M, Kasa P, Janka Z, Kalman J. ipramine and citalopram facilitate amyloid precursor protein secretion in vitro. Neurochem Int. 2005; 47(3):190–195. PMID: 15955598.23. Pennington PR, Wei Z, Rui L, Doig JA, Graham B, Kuski K, Gabriel GG, Mousseau DD. Alzheimer disease-related presenilin-1 variants exert distinct effects on monoamine oxidase-A activity in vitro. J Neural Transm. 2011; 118(7):987–995. PMID: 21373759.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peroxiredoxin I regulates the component expression of gamma-secretase complex causing the Alzheimer's disease
- Chronic Reserpine Administration for Depression Modeling in Zebrafish
- Overexpression of N141I PS2 increases γ-secretase activity through up-regulation of Presenilin and Pen-2 in brain mitochondria of NSE/hPS2m transgenic mice
- Baclofen Abuse due to Its Hypomanic Effect in Patients with Alcohol Dependence and Comorbid Major Depressive Disorder
- Effects of Red Liriope platyphylla on NGF secretion ability, NGF receptor signaling pathway and gamma-secretase components in NSE/hAPPsw transgenic mice expressing Alzheimer's Disease