Korean J Physiol Pharmacol.
2013 Jun;17(3):209-216. 10.4196/kjpp.2013.17.3.209.
Protective Effect of Phosphatidylcholine on Lipopolysaccharide-Induced Acute Inflammation in Multiple Organ Injury
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea. jhjeong3@cau.ac.kr
- 2Department of Pathology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea.
- 3Department of Anatomy, College of Medicine, Chung-Ang University, Seoul 156-756, Korea.
- KMID: 1429300
- DOI: http://doi.org/10.4196/kjpp.2013.17.3.209
Abstract
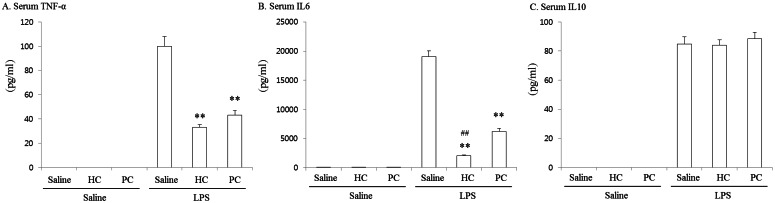
- Soybean polyunsaturated phosphatidylcholine (PC) is thought to exert anti-inflammatory activities and has potent effects in attenuating acute renal failure and liver dysfunction. The aim of this study was to investigate the effects of PC in protecting multiple organ injury (MOI) from lipopolysaccharide (LPS). Six groups of rats (N=8) were used in this study. Three groups acted as controls and received only saline, hydrocortisone (HC, 6 mg/kg, i.v.) or PC (600 mg/kg, i.p.) without LPS (15 mg/kg, i.p.) injections. Other 3 groups, as the test groups, were administered saline, HC or PC in the presence of LPS. Six hours after the LPS injection, blood and organs (lung, liver and kidney) were collected from each group to measure inflammatory cytokines and perform histopathology and myeloperoxidase (MPO) assessment. Serum cytokines (TNF-alpha, IL-6 and IL-10) and MPO activities were significantly increased, and significant histopathological changes in the organs were observed by LPS challenge. These findings were significantly attenuated by PC or HC. The treatment with PC or HC resulted in a significant attenuation on the increase in serum levels of TNF-alpha and IL-6, pro-inflammatory cytokines, while neither PC nor HC significantly attenuated serum levels of IL-10, anti-inflammatory cytokine. In the organs, the enhanced infiltration of neutrophils and expression of ED2 positive macrophage were attenuated by PC or HC. Inductions of MPO activity were also significantly attenuated by PC or HC. From the findings, we suggest that PC may be a functional material for its use as an anti-inflammatory agent.
Keyword
MeSH Terms
-
Acute Kidney Injury
Animals
Cytokines
Hydrocortisone
Inflammation
Interleukin-10
Interleukin-6
Kidney
Liver
Liver Diseases
Lung
Macrophages
Neutrophils
Peroxidase
Phosphatidylcholines
Rats
Soybeans
Tumor Necrosis Factor-alpha
Cytokines
Hydrocortisone
Interleukin-10
Interleukin-6
Peroxidase
Phosphatidylcholines
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
Scant Extracellular NAD Cleaving Activity of Human Neutrophils is Down-Regulated by fMLP via FPRL1
Md. Ashraful Hasan, Md. Tipu Sultan, Won-Gyun Ahn, Yeon-Ja Kim, Ji-Hye Jang, Chang-Won Hong, Dong-Keun Song
Korean J Physiol Pharmacol. 2014;18(6):497-502. doi: 10.4196/kjpp.2014.18.6.497.
Reference
-
1. Proulx F, Joyal JS, Mariscalco MM, Leteurtre S, Leclerc F, Lacroix J. The pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2009; 10:12–22. PMID: 19057438.
Article2. Papathanassoglou ED, Bozas E, Giannakopoulou MD. Multiple organ dysfunction syndrome pathogenesis and care: a complex systems' theory perspective. Nurs Crit Care. 2008; 13:249–259. PMID: 18816311.
Article3. Butt I, Shrestha BM. Two-hit hypothesis and multiple organ dysfunction syndrome. JNMA J Nepal Med Assoc. 2008; 47:82–85. PMID: 18709037.
Article4. Krau SD. Making sense of multiple organ dysfunction syndrome. Crit Care Nurs Clin North Am. 2007; 19:87–97. PMID: 17338954.
Article5. Mizock BA. The multiple organ dysfunction syndrome. Dis Mon. 2009; 55:476–526. PMID: 19595297.
Article6. Lipsky M. Multiple organ dysfunction syndrome. Foreword. Dis Mon. 2009; 55:475. PMID: 19595296.7. Johnson D, Mayers I. Multiple organ dysfunction syndrome: a narrative review. Can J Anaesth. 2001; 48:502–509. PMID: 11394523.
Article8. Wadhwa J, Sood R. Multiple organ dysfunction syndrome. Natl Med J India. 1997; 10:277–282. PMID: 9481099.9. Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996; 60:316–341. PMID: 8801436.
Article10. Henderson B, Wilson M. Modulins: a new class of cytokine-inducing, pro-inflammatory bacterial virulence factor. Inflamm Res. 1995; 44:187–197. PMID: 7655992.
Article11. Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000; 13:110–116. PMID: 10670840.
Article12. Bone RC. Sepsis, the sepsis syndrome, multi-organ failure: a plea for comparable definitions. Ann Intern Med. 1991; 114:332–333. PMID: 1987879.
Article13. Bohlinger I, Leist M, Gantner F, Angermüller S, Tiegs G, Wendel A. DNA fragmentation in mouse organs during endotoxic shock. Am J Pathol. 1996; 149:1381–1393. PMID: 8863685.14. Mourelle M, Guarner F, Malagelada JR. Polyunsaturated phosphatidylcholine prevents stricture formation in a rat model of colitis. Gastroenterology. 1996; 110:1093–1097. PMID: 8612998.
Article15. Fallbrook A, Turenne SD, Mamalias N, Kish SJ, Ross BM. Phosphatidylcholine and phosphatidylethanolamine metabolites may regulate brain phospholipid catabolism via inhibition of lysophospholipase activity. Brain Res. 1999; 834:207–210. PMID: 10407117.
Article16. Blusztajn JK, Zeisel SH, Wurtman RJ. Synthesis of lecithin (phosphatidylcholine) from phosphatidylethanolamine in bovine brain. Brain Res. 1979; 179:319–327. PMID: 509240.
Article17. Cheatham CL, Goldman BD, Fischer LM, da Costa KA, Reznick JS, Zeisel SH. Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2012; 96:1465–1472. PMID: 23134891.
Article18. Chung SY, Moriyama T, Uezu E, Uezu K, Hirata R, Yohena N, Masuda Y, Kokubu T, Yamamoto S. Administration of phosphatidylcholine increases brain acetylcholine concentration and improves memory in mice with dementia. J Nutr. 1995; 125:1484–1489. PMID: 7782901.19. Rey JW, Schreiner O, Barreiros AP, Heise M, Krupp M, Schuchmann M, Otto G, Galle PR, Teufel A. Acute renal failure and liver dysfunction after subcutaneous injection of 3-snphosphatidylcholine (Lipostabil®)-case report. Z Gastroenterol. 2011; 49:340–343. PMID: 21391165.20. Ghyczy M, Torday C, Kaszaki J, Szabó A, Czóbel M, Boros M. Oral phosphatidylcholine pretreatment decreases ischemia-reperfusion-induced methane generation and the inflammatory response in the small intestine. Shock. 2008; 30:596–602. PMID: 18461026.
Article21. Al-Orf SM. Effect of oxidized phosphatidylcholine on biomarkers of oxidative stress in rats. Indian J Clin Biochem. 2011; 26:154–160. PMID: 22468042.
Article22. Tokés T, Eros G, Bebes A, Hartmann P, Várszegi S, Varga G, Kaszaki J, Gulya K, Ghyczy M, Boros M. Protective effects of a phosphatidylcholine-enriched diet in lipopolysaccharide-induced experimental neuroinflammation in the rat. Shock. 2011; 36:458–465. PMID: 21937953.23. Dial EJ, Zayat M, Lopez-Storey M, Tran D, Lichtenberger L. Oral phosphatidylcholine preserves the gastrointestinal mucosal barrier during LPS-induced inflammation. Shock. 2008; 30:729–733. PMID: 18496240.
Article24. Orr SK, Trépanier MO, Bazinet RP. n-3 Polyunsaturated fatty acids in animal models with neuroinflammation. Prostaglandins Leukot Essent Fatty Acids. 2013; 88:97–103. PMID: 22770766.
Article25. Das UN. Infection, inflammation, and polyunsaturated fatty acids. Nutrition. 2011; 27:1080–1084. PMID: 21907900.
Article26. Tandy S, Chung RW, Kamili A, Wat E, Weir JM, Meikle PJ, Cohn JS. Hydrogenated phosphatidylcholine supplementation reduces hepatic lipid levels in mice fed a high-fat diet. Atherosclerosis. 2010; 213:142–147. PMID: 20832797.
Article27. Polfliet MM, Fabriek BO, Daniëls WP, Dijkstra CD, van den Berg TK. The rat macrophage scavenger receptor CD163: expression, regulation and role in inflammatory mediator production. Immunobiology. 2006; 211:419–425. PMID: 16920481.
Article28. Englert JA, Fink MP. The multiple organ dysfunction syndrome and late-phase mortality in sepsis. Curr Infect Dis Rep. 2005; 7:335–341. PMID: 16107229.
Article29. Alves C, Robazzi TC, Mendonça M. Withdrawal from glucocorticosteroid therapy: clinical practice recommendations. J Pediatr (Rio J). 2008; 84:192–202. PMID: 18535733.
Article30. Annane D. Corticosteroids for septic shock. Crit Care Med. 2001; 29(7 Suppl):S117–S120. PMID: 11445745.
Article31. Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995; 23:1294–1303. PMID: 7600840.32. Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, Fisher CJ Jr. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995; 23:1430–1439. PMID: 7634816.33. van der Poll T, Barber AE, Coyle SM, Lowry SF. Hypercortisolemia increases plasma interleukin-10 concentrations during human endotoxemia--a clinical research center study. J Clin Endocrinol Metab. 1996; 81:3604–3606. PMID: 8855809.
Article34. Briegel J, Kellermann W, Forst H, Haller M, Bittl M, Hoffmann GE, Büchler M, Uhl W, Peter K. The Phospholipase A2 Study Group. Low-dose hydrocortisone infusion attenuates the systemic inflammatory response syndrome. Clin Investig. 1994; 72:782–787.
Article35. Osman MO, Jacobsen NO, Kristensen JU, Larsen CG, Jensen SL. Beneficial effects of hydrocortisone in a model of experimental acute pancreatitis. Dig Surg. 1999; 16:214–221. PMID: 10436370.
Article36. Hartmann P, Szabó A, Eros G, Gurabi D, Horváth G, Németh I, Ghyczy M, Boros M. Anti-inflammatory effects of phosphatidylcholine in neutrophil leukocyte-dependent acute arthritis in rats. Eur J Pharmacol. 2009; 622:58–64. PMID: 19766625.
Article37. Eros G, Varga G, Váradi R, Czóbel M, Kaszaki J, Ghyczy M, Boros M. Anti-inflammatory action of a phosphatidylcholine, phosphatidylethanolamine and N-acylphosphatidylethanolamine-enriched diet in carrageenan-induced pleurisy. Eur Surg Res. 2009; 42:40–48. PMID: 18987473.
Article38. Aono K, Isobe K, Kiuchi K, Fan ZH, Ito M, Takeuchi A, Miyachi M, Nakashima I, Nimura Y. In vitro and in vivo expression of inducible nitric oxide synthase during experimental endotoxemia: involvement of other cytokines. J Cell Biochem. 1997; 65:349–358. PMID: 9138091.39. Riches DW, Chan ED, Winston BW. TNF-alpha-induced regulation and signalling in macrophages. Immunobiology. 1996; 195:477–490. PMID: 8933152.40. Qiu P, Cui X, Barochia A, Li Y, Natanson C, Eichacker PQ. The evolving experience with therapeutic TNF inhibition in sepsis: considering the potential influence of risk of death. Expert Opin Investig Drugs. 2011; 20:1555–1564.
Article41. Song R, Kim J, Yu D, Park C, Park J. Kinetics of IL-6 and TNF-α changes in a canine model of sepsis induced by endotoxin. Vet Immunol Immunopathol. 2012; 146:143–149. PMID: 22424937.
Article42. Oda S, Hirasawa H, Shiga H, Nakanishi K, Matsuda K, Nakamua M. Sequential measurement of IL-6 blood levels in patients with systemic inflammatory response syndrome (SIRS)/sepsis. Cytokine. 2005; 29:169–175. PMID: 15652449.
Article43. Leon LR, White AA, Kluger MJ. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol. 1998; 275:R269–R277. PMID: 9688988.
Article44. Neri M, Bello S, Bonsignore A, Centini F, Fiore C, Földes-Papp Z, Turillazzi E, Fineschi V. Myocardial expression of TNFalpha, IL-1beta, IL-6, IL-8, IL-10 and MCP-1 after a single MDMA dose administered in a rat model. Curr Pharm Biotechnol. 2010; 11:413–420. PMID: 20420568.45. Refsum SE, Halliday MI, Campbell G, McCaigue M, Rowlands BJ, Boston VE. Modulation of TNF alpha and IL-6 in a peritonitis model using pentoxifylline. J Pediatr Surg. 1996; 31:928–930. PMID: 8811559.46. Engelberts I, von Asmuth EJ, van der Linden CJ, Buurman WA. The interrelation between TNF, IL-6, and PAF secretion induced by LPS in an in vivo and in vitro murine model. Lymphokine Cytokine Res. 1991; 10:127–131. PMID: 1873355.47. Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train. 2006; 41:457–465. PMID: 17273473.48. Reumaux D, de Boer M, Meijer AB, Duthilleul P, Roos D. Expression of myeloperoxidase (MPO) by neutrophils is necessary for their activation by anti-neutrophil cytoplasm autoantibodies (ANCA) against MPO. J Leukoc Biol. 2003; 73:841–849. PMID: 12773517.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ventilator-Induced Lung Injury
- Brain consequences of acute kidney injury: Focusing on the hippocampus
- Diosmetin Alleviates Lipopolysaccharide-Induced Acute Lung Injury through Activating the Nrf2 Pathway and Inhibiting the NLRP3 Inflammasome
- Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress
- Contrast-induced Acute Kidney Injury and Inflammation