Nutr Res Pract.
2023 Dec;17(6):1043-1055. 10.4162/nrp.2023.17.6.1043.
Cydonia oblonga Miller fruit extract exerts an anti-obesity effect in 3T3-L1 adipocytes by activating the AMPK signaling pathway
- Affiliations
-
- 1Department of Food Science & Nutrition, Dongseo University, Busan 47011, Korea
- 2Industry coupled Cooperation Center for Bio Healthcare Materials, Hallym University, Chuncheon 24252, Korea
- 3Research Institute, BnG Inc., Chuncheon 24232, Korea
- KMID: 2548863
- DOI: http://doi.org/10.4162/nrp.2023.17.6.1043
Abstract
- BACKGROUND/OBJECTIVES
The fruit of Cydonia oblonga Miller (COM) is used traditionally in Mediterranean region medicine to prevent or treat obesity, but its mechanism of action is still unclear. Beyond a demonstrated anti-obesity effect, the fruit was tested for the mechanism of adipogenesis in 3T3-L1 preadipocytes.
MATERIALS/METHODS
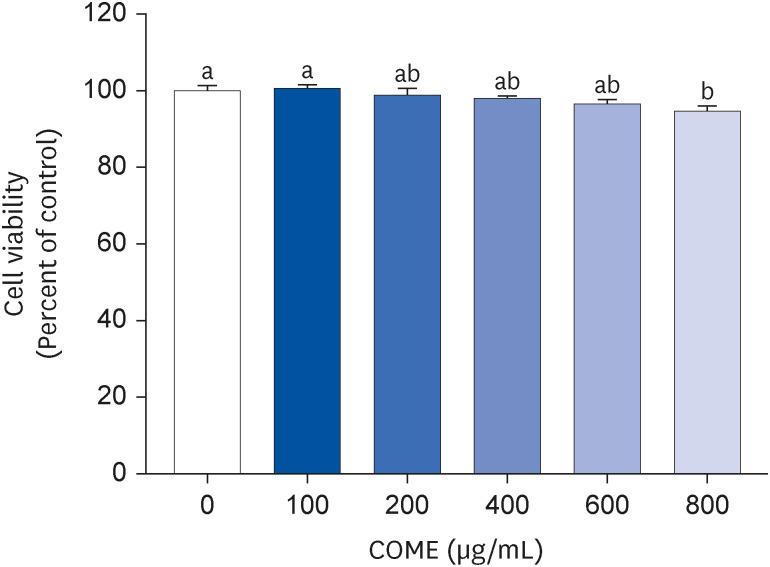
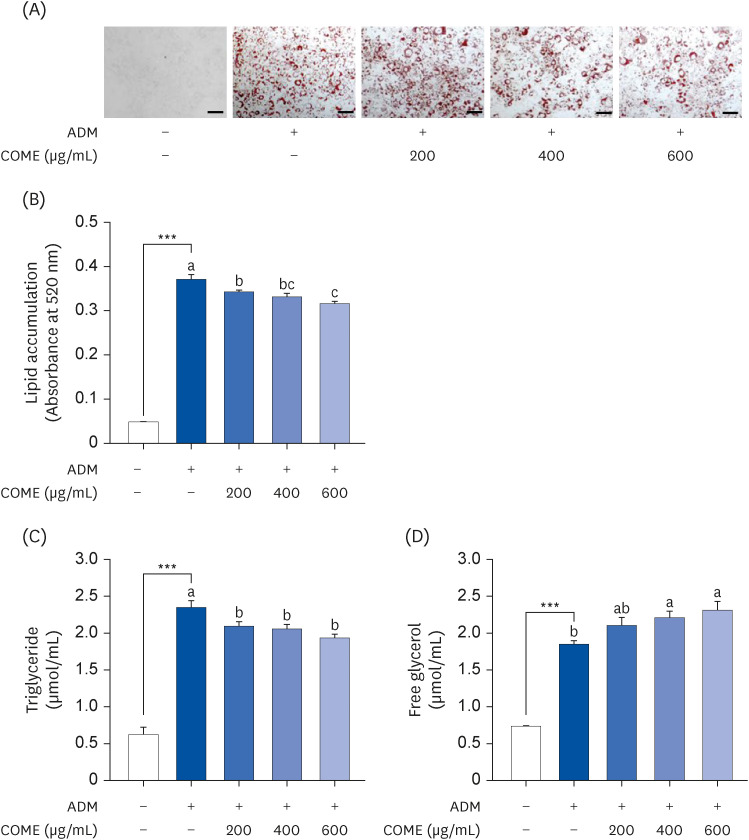
3T3-L1 preadipocytes were cultured for 8 days with COM fruit extract (COME) at different concentrations (0–600 µg/mL) with adipocyte differentiation medium. The cell viability was measured using an MTT assay; triglyceride (TG) was stained with Oil Red O. The expression levels of the adipogenesis-related genes and protein expression were analyzed by reverse transcription polymerase chain reaction and Western blotting, respectively.
RESULTS
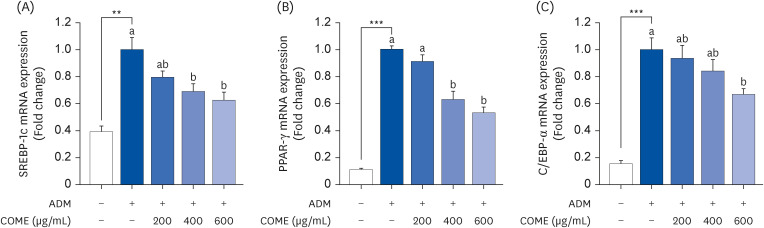
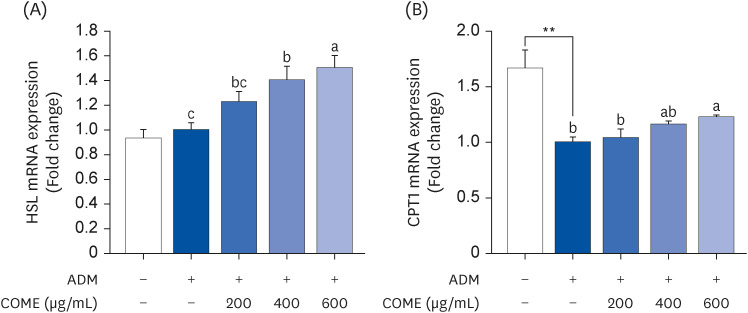
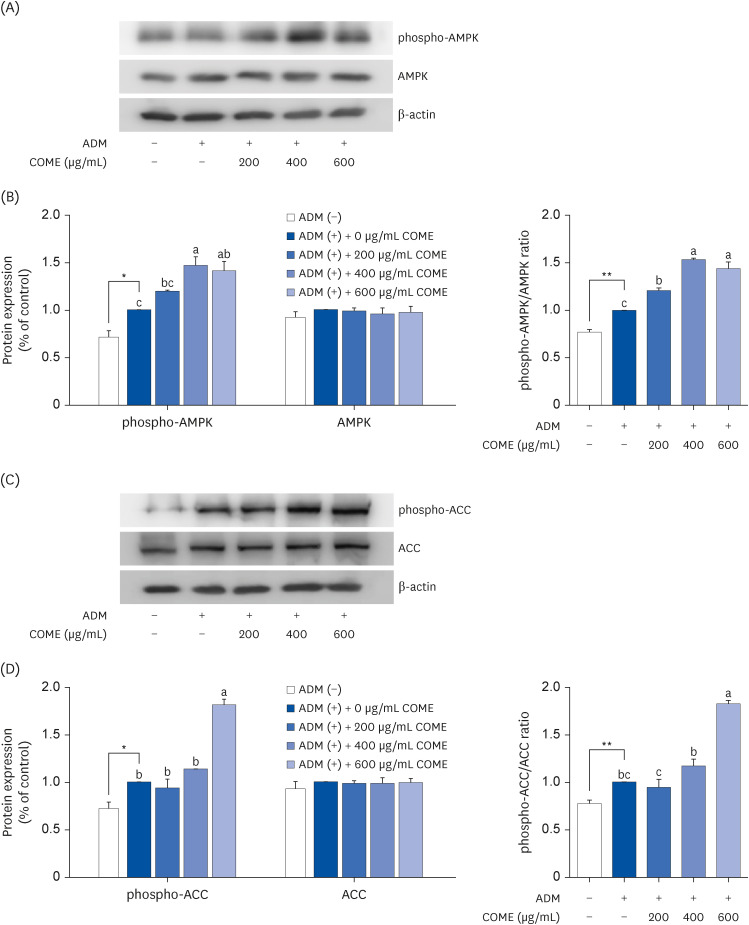
COME inhibited intracellular TG accumulation during adipogenesis. A COME treatment in 3T3-L1 cells induced upregulation of the adenosine monophosphateactivated protein kinase (AMPK)α phosphorylation and downregulation of the adipogenic transcription factors, such as sterol regulatory element-binding protein 1c, peroxisome proliferator-activated receptor γ, and CCAAT/enhancer binding protein α. The COME treatment reduced the mRNA expression of fatty acyl synthetase, adenosine triphosphatecitrate lyase, adipocyte protein 2, and lipoprotein lipase. It increased the mRNA expression of hormone-sensitive lipase and carnitine palmitoyltransferase I in 3T3-L1 cells.
CONCLUSIONS
COME inhibits adipogenesis via the AMPK signaling pathways. COME may be used to prevent and treat obesity.
Figure
Reference
-
1. Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, Milani RV. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018; 61:142–150. PMID: 29981771.2. Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. 2015; 66(Suppl 2):7–12.3. Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res. 2011; 50:14–27. PMID: 21087632.4. Saponaro C, Gaggini M, Carli F, Gastaldelli A. The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients. 2015; 7:9453–9474. PMID: 26580649.5. Adamczak M, Wiecek A. The adipose tissue as an endocrine organ. Semin Nephrol. 2013; 33:2–13. PMID: 23374889.6. Poojashree MJ, Siddalingaprasad HS, Swetha BR, Shivukumar S. A review on the current drugs and new targets for obesity. J Appl Pharm Res. 2020; 8:11–21.7. Baretić M. Obesity drug therapy. Minerva Endocrinol. 2013; 38:245–254. PMID: 24126545.8. Kang JG, Park CY. Anti-obesity drugs: a review about their effects and safety. Diabetes Metab J. 2012; 36:13–25. PMID: 22363917.9. Hayamizu K, Ishii Y, Kaneko I, Shen M, Okuhara Y, Shigematsu N, Tomi H, Furuse M, Yoshino G, Shimasaki H. Effects of garcinia cambogia (hydroxycitric acid) on visceral fat accumulation: a double-blind, randomized, placebo-controlled trial. Curr Ther Res Clin Exp. 2003; 64:551–567. PMID: 24944404.10. Semwal RB, Semwal DK, Vermaak I, Viljoen A. A comprehensive scientific overview of Garcinia cambogia . Fitoterapia. 2015; 102:134–148. PMID: 25732350.11. Klaus S, Pültz S, Thöne-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes. 2005; 29:615–623.12. Wang H, Wen Y, Du Y, Yan X, Guo H, Rycroft JA, Boon N, Kovacs EM, Mela DJ. Effects of catechin enriched green tea on body composition. Obesity (Silver Spring). 2010; 18:773–779. PMID: 19680234.13. Abdollahi H. A review on history, domestication and germplasm collections of quince (Cydonia oblonga Mill.) in the world. Genet Resour Crop Evol. 2019; 66:1041–1058.14. Zhou WT, Abdurahman A, Abdusalam E, Yiming W, Abliz P, Aji Q, Issak M, Iskandar G, Moore N, Umar A. Effect of Cydonia oblonga Mill. leaf extracts or captopril on blood pressure and related biomarkers in renal hypertensive rats. J Ethnopharmacol. 2014; 153:635–640. PMID: 24661965.15. Ashraf MU, Muhammad G, Hussain MA, Bukhari SNA. Cydonia oblonga M., A medicinal plant rich in phytonutrients for pharmaceuticals. Front Pharmacol. 2016; 7:163. PMID: 27445806.16. Silva BM, Andrade PB, Ferreres F, Domingues AL, Seabra RM, Ferreira MA. Phenolic profile of quince fruit (Cydonia oblonga Miller) (pulp and peel). J Agric Food Chem. 2002; 50:4615–4618. PMID: 12137485.17. Wojdyło A, Oszmiański J, Bielicki P. Polyphenolic composition, antioxidant activity, and polyphenol oxidase (PPO) activity of quince (Cydonia oblonga Miller) varieties. J Agric Food Chem. 2013; 61:2762–2772. PMID: 23461298.18. Essafi-Benkhadir K, Refai A, Riahi I, Fattouch S, Karoui H, Essafi M. Quince (Cydonia oblonga Miller) peel polyphenols modulate LPS-induced inflammation in human THP-1-derived macrophages through NF-κB, p38MAPK and Akt inhibition. Biochem Biophys Res Commun. 2012; 418:180–185. PMID: 22252293.19. Pacifico S, Gallicchio M, Fiorentino A, Fischer A, Meyer U, Stintzing FC. Antioxidant properties and cytotoxic effects on human cancer cell lines of aqueous fermented and lipophilic quince (Cydonia oblonga Mill.) preparations. Food Chem Toxicol. 2012; 50:4130–4135. PMID: 23034449.20. Adiban H, Shirazi FH, Gholami S, Kamalinejad M, Hosseini SH, Noubarani M, Eskandari MR. Chemopreventive effect of quince (Cydonia oblonga Mill.) fruit extract on hepatocellular carcinoma induced by diethylnitrosamine in rats. Int Pharm Acta. 2019; 2:e2.21. Mirmohammadlu M, Hosseini SH, Kamalinejad M, Esmaeili Gavgani M, Noubarani M, Eskandari MR. Hypolipidemic, hepatoprotective and renoprotective effects of Cydonia oblonga Mill. fruit in streptozotocin-induced diabetic rats. Iran J Pharm Res. 2015; 14:1207–1214. PMID: 26664388.22. Zhou W, Abdusalam E, Abliz P, Reyim N, Tian S, Aji Q, Issak M, Iskandar G, Moore N, Umar A. Effect of Cydonia oblonga Mill. fruit and leaf extracts on blood pressure and blood rheology in renal hypertensive rats. J Ethnopharmacol. 2014; 152:464–469. PMID: 24472663.23. Umar A, Iskandar G, Aikemu A, Yiming W, Zhou W, Berké B, Begaud B, Moore N. Effects of Cydonia oblonga Miller leaf and fruit flavonoids on blood lipids and anti-oxydant potential in hyperlipidemia rats. J Ethnopharmacol. 2015; 169:239–243. PMID: 25934516.24. Lee HS, Jeon YE, Jung JI, Kim SM, Hong SH, Lee J, Hwang JS, Hwang MO, Kwon K, Kim EJ. Anti-obesity effect of Cydonia oblonga Miller extract in high-fat diet-induced obese C57BL/6 mice. J Funct Foods. 2022; 89:104945.25. Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986; 89:271–277. PMID: 3486233.26. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509. PMID: 13428781.27. Lim SM, Jung JI, Kim NY, Bae JS, Lee HS, Kim EJ. Cyanidine-3-O-galactoside enriched Aronia melanocarpa extract inhibits adipogenesis and lipogenesis via down-regulation of adipogenic transcription factors and their target genes in 3T3-L1 Cells. Food Nutr Sci. 2019; 10:128–147.28. Kim YH, Jung JI, Jeon YE, Kim SM, Oh TK, Lee J, Moon JM, Kim TY, Kim EJ. Gynostemma pentaphyllum extract and Gypenoside L enhance skeletal muscle differentiation and mitochondrial metabolism by activating the PGC-1α pathway in C2C12 myotubes. Nutr Res Pract. 2022; 16:14–32. PMID: 35116125.29. Guru A, Issac PK, Velayutham M, Saraswathi NT, Arshad A, Arockiaraj J. Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds. Mol Biol Rep. 2021; 48:743–761. PMID: 33275195.30. Jemai R, Drira R, Makni M, Fetoui H, Sakamoto K. Colocynth (Citrullus colocynthis) seed extracts attenuate adipogenesis by down-regulating PPARγ/ SREBP-1c and C/EBPα in 3T3-L1 cells. Food Biosci. 2020; 33:100491.31. Khan N, Maihemuti N, Nuer M, Abudurousuli K, Simayi J, Talihati Z, Han M, Hailati S, Zhou W, Wumaier A. Analysis of major polyphenolic compounds of Cydonia oblonga Miller (Quince) fruit extract by UPLC-MS/MS and its effect on adipogenesis in 3T3-L1 cells. Separations. 2022; 9:167.32. Ko JH, Kwon HS, Yoon JM, Yoo JS, Jang HS, Kim JY, Yeon SW, Kang JH. Effects of Polygonatum sibiricum rhizome ethanol extract in high-fat diet-fed mice. Pharm Biol. 2015; 53:563–570. PMID: 25327577.33. Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci U S A. 2003; 100:12027–12032. PMID: 14512514.34. Gambero A, Ribeiro ML. The positive effects of yerba maté (Ilex paraguariensis) in obesity. Nutrients. 2015; 7:730–750. PMID: 25621503.35. Wong RHF, Sul HS. Insulin signaling in fatty acid and fat synthesis: a transcriptional perspective. Curr Opin Pharmacol. 2010; 10:684–691. PMID: 20817607.36. Guay C, Madiraju SRM, Aumais A, Joly E, Prentki M. A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J Biol Chem. 2007; 282:35657–35665. PMID: 17928289.37. Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, Cao Q, Atsumi G, Malone H, Krishnan B, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005; 1:107–119. PMID: 16054052.38. de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008; 54:945–955. PMID: 18436717.39. Mukherjee B, Hossain CM, Mondal L, Paul P, Ghosh MK. Obesity and insulin resistance: an abridged molecular correlation. Lipid Insights. 2013; 6:1–11. PMID: 25278764.40. Woods A, Williams JR, Muckett PJ, Mayer FV, Liljevald M, Bohlooly-Y M, Carling D. Liver-specific activation of AMPK prevents steatosis on a high-fructose diet. Cell Reports. 2017; 18:3043–3051. PMID: 28355557.41. Lee J, Park S, Oh N, Park J, Kwon M, Seo J, Roh S. Oral intake of Lactobacillus plantarum L-14 extract alleviates TLR2- and AMPK-mediated obesity-associated disorders in high-fat-diet-induced obese C57BL/6J mice. Cell Prolif. 2021; 54:e13039. PMID: 33830560.42. Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016; 48:e245. PMID: 27416781.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- The Effect of AMPK Activation on Wnt and sFRP5 in TNF-alpha Induced Adipocyte Metabolic Dysfunction in 3T3-L1 Cell
- The inhibition of inflammatory molecule expression on 3T3-L1 adipocytes by berberine is not mediated by leptin signaling
- Carnosic acid inhibits TLR4-MyD88 signaling pathway in LPS-stimulated 3T3-L1 adipocytes
- Nelumbo nucifera Leaf Extract Regulates Lipid Metabolism and Differentiation in 3T3-L1 Adipocytes and db/db Mice