Nutr Res Pract.
2014 Feb;8(1):33-39.
Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- Affiliations
-
- 1Department of Food & Nutrition, Mokpo National University, 1666 Yeongsan-ro, Cheonggye-myeon, Muan-gun, Jeonnam 534-729, Korea. kha@mokpo.ac.kr
- 2Jeonnam Biofood Technology Center, Naju, Korea.
Abstract
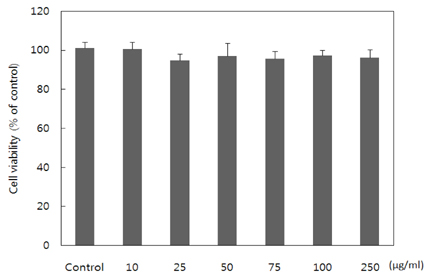
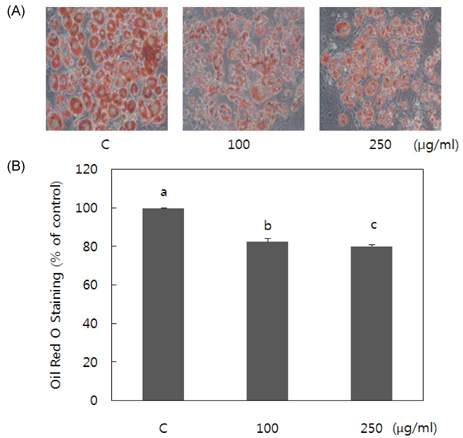
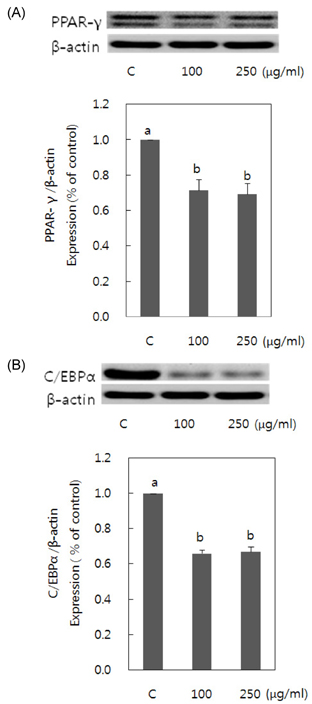
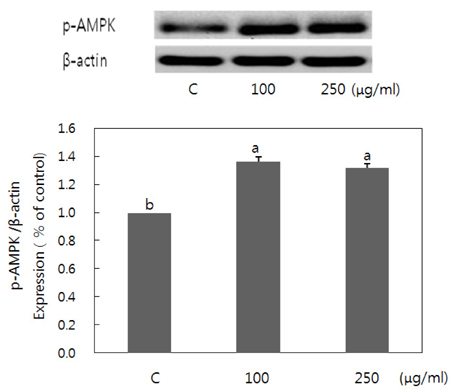
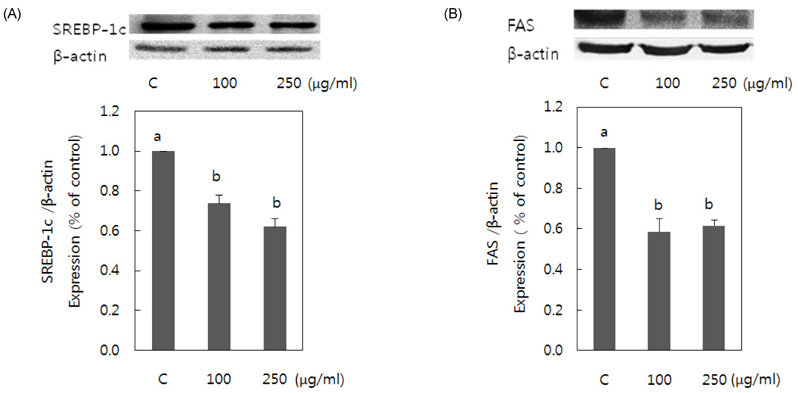
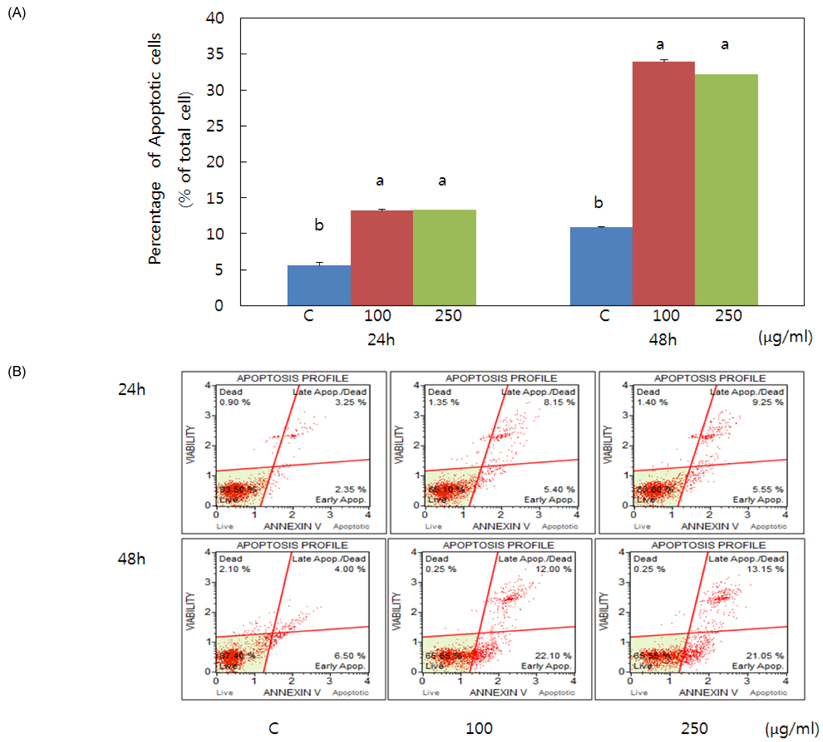
- Obesity occurs when a person's calorie intake exceeds the amount of energy burns, which may lead to pathologic growth of adipocytes and the accumulation of fat in the tissues. In this study, the effect and mechanism of pear pomace extracts on 3T3-L1 adipocyte differentiation and apoptosis of mature adipocytes were investigated. The effects of pear pomace extract on cell viability and the anti-adipogenic and proapoptotic effects were investigated via MTT assay, Oil red O staining, western blot analysis and apoptosis assay. 3T3-L1 preadipocytes were stimulated with DMEM containing 10% FBS, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 5 microg/ml insulin and 1 microM dexamethasone for differentiation to adipocytes. 3T3-L1 cells were cultured with PBS or water extract of pear pomace. Water extract of pear pomace effectively inhibited lipid accumulations and expressions of PPAR-gamma and C/EBPalpha in 3T3-L1 cells. It also increased expression of p-AMPK and decreased the expression of SREBP-1c and FAS in 3T3-L1 cells. The induction of apoptosis was observed in 3T3-L1 cells treated with pear pomace. These results indicate that pear pomace water extract inhibits adipogenesis and induces apoptosis of adipocytes and thus can be used as a potential therapeutic substance as part of prevention or treatment strategy for obesity.
MeSH Terms
Figure
Reference
-
1. Cowherd RM, Lyle RE, McGehee RE Jr. Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. 1999; 10:3–10.
Article2. Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007; 131:242–256.
Article3. Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999; 4:611–617.
Article4. Hamm JK, Park BH, Farmer SR. A role for C/EBPbeta in regulating peroxisome proliferator-activated receptor gamma activity during adipogenesis in 3T3-L1 preadipocytes. J Biol Chem. 2001; 276:18464–18471.
Article5. Erbayraktar Z, Yilmaz O, Artmann AT, Cehreli R, Coker C. Effects of selenium supplementation on antioxidant defense and glucose homeostasis in experimental diabetes mellitus. Biol Trace Elem Res. 2007; 118:217–226.
Article6. Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002; 277:25226–25232.
Article7. Viollet B, Andreelli F, Jørgensen SB, Perrin C, Flamez D, Mu J, Wojtaszewski JF, Schuit FC, Birnbaum M, Richter E, Burcelin R, Vaulont S. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem Soc Trans. 2003; 31:216–219.
Article8. Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007; 100:328–341.
Article9. Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem. 2008; 283:16514–16524.
Article10. Morrison RF, Farmer SR. Hormonal signaling and transcriptional control of adipocyte differentiation. J Nutr. 2000; 130:3116S–3121S.
Article11. Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006; 7:885–896.
Article12. Zhang X, Koo J, Eun JB. Antioxidant acrivities of metnanol extracts and phenolic compounds in Asian pear at different stages of maturity. Food Sci Biotechnol. 2006; 15:44–50.13. Zhang X, Na CS, Kim JS, Lee FZ, Eun JB. Changes in dietary fiber content of flesh and peel in three cultivars of Asian pears during growth. Food Sci Biotechnol. 2003; 12:358–364.14. Kim JS, Na CS. Effects of pear phenolic compound on the STZ-treated mice for induction of diabetes. J Korean Soc Food Sci Nutr. 2002; 31:1107–1111.
Article15. Na CS, Youn DH, Choi DH, Jeong JG, Eun JB, Kim JS. The effect of pear pectin & phenolic compounds on regional cerebral blood flow, mean arterial blood pressure, heart rate and cardiac contractile force in hypertensive rat induced by 2K1C. Korean J Herbol. 2003; 18:101–108.16. Lee I, Kim J, Ryoo I, Kim Y, Choo S, Yoo I, Min B, Na M, Hattori M, Bae K. Lanostane triterpenes from Ganoderma lucidum suppress the adipogenesis in 3T3-L1 cells through down-regulation of SREBP-1c. Bioorg Med Chem Lett. 2010; 20:5577–5581.
Article17. Green H, Kehinde O. Sublines of mouse 3T3 cells that accumulate lipid. Cell. 1974; 1:113–116.
Article18. Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974; 3:127–133.
Article19. Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975; 5:19–27.
Article20. Tang JJ, Li JG, Qi W, Qiu WW, Li PS, Li BL, Song BL. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab. 2011; 13:44–56.
Article21. Krycer JR, Sharpe LJ, Luu W, Brown AJ. The Akt-SREBP nexus: cell signaling meets lipid metabolism. Trends Endocrinol Metab. 2010; 21:268–276.
Article22. Viollet B, Guigas B, Leclerc J, Hébrard S, Lantier L, Mounier R, Andreelli F, Foretz M. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf). 2009; 196:81–98.
Article23. Park SY, Hwang JT, Lee YK, Kim YM, Park OJ. AMP-activated kinase regulates adipocyte differentiation process in 3T3-L1 adipocytes treated with selenium. J Life Sci. 2009; 19:423–428.
Article24. Hsu CL, Huang SL, Yen GC. Inhibitory effect of phenolic acids on the proliferation of 3T3-L1 preadipocytes in relation to their antioxidant activity. J Agric Food Chem. 2006; 54:4191–4197.
Article25. Hsu CL, Yen GC. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 cells. J Agric Food Chem. 2007; 55:1730–1736.
Article26. Kola B. Role of AMP-activated protein kinase in the control of appetite. J Neuroendocrinol. 2008; 20:942–951.
Article27. Kola B, Grossman AB, Korbonits M. The role of AMP-activated protein kinase in obesity. Front Horm Res. 2008; 36:198–211.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The micosporine-like amino acids-rich aqueous methanol extract of laver (Porphyra yezoensis) inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- Soluble extract of soybean fermented with Aspergillus oryzae GB107 inhibits fat accumulation in cultured 3T3-L1 adipocytes
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3