Nutr Res Pract.
2014 Oct;8(5):516-520. 10.4162/nrp.2014.8.5.516.
Carnosic acid inhibits TLR4-MyD88 signaling pathway in LPS-stimulated 3T3-L1 adipocytes
- Affiliations
-
- 1Department of Food & Nutrition Education, Graduate School of Education, Soonchunhyang University, Asan, Chungnam 336-745, Korea.
- 2Department of Obstetrics and Gynecology, Soonchunhyang University Cheonan Hospital, Soonchunhyang-6-gil 31, Dongnam-gu, Cheonan 330-721, Korea. sternum@schmc.ac.kr
- KMID: 2313772
- DOI: http://doi.org/10.4162/nrp.2014.8.5.516
Abstract
- BACKGROUND/OBJECTIVES
Carnosic acid (CA), found in rosemary (Rosemarinus officinalis) leaves, is known to exhibit anti-obesity and anti-inflammatory activities. However, whether its anti-inflammatory potency can contribute to the amelioration of obesity has not been elucidated. The aim of the current study was to investigate the effect of CA on Toll-like receptor 4 (TLR4) pathways in the presence of lipopolysaccharide (LPS) in 3T3-L1 adipocytes.
MATERIALS/METHODS
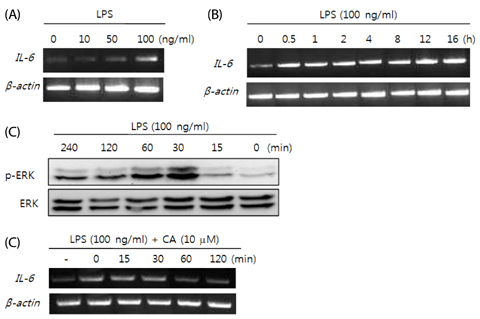
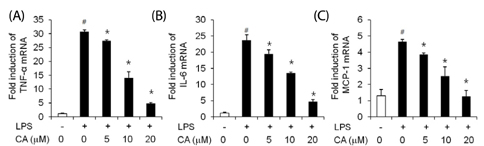
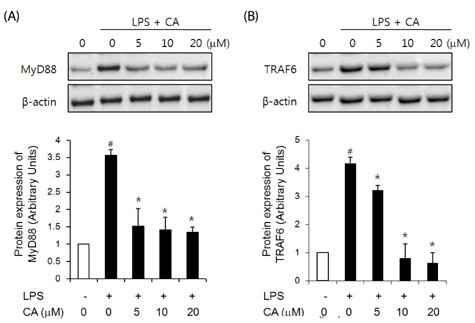
3T3-L1 adipocytes were treated with CA (0-20 microM) for 1 h, followed by treatment with LPS for 30 min; mRNA expression of adipokines and protein expression of TLR4-related molecules were then measured.
RESULTS
LPS-stimulated 3T3-L1 adipocytes showed elevated mRNA expression of tumor necrosis factor (TNF)-alpha, interleukin-6, and monocyte chemoattractant protein-1, and CA significantly inhibited the expression of these adipokine genes. LPS-induced up regulation of TLR4, myeloid differentiation factor 88, TNF receptor-associated factor 6, and nuclear factor-kappaB, as well as phosphorylated extracellular receptor-activated kinase were also suppressed by pre-treatment of 3T3-L1 adipocytes with CA.
CONCLUSIONS
Results of this study suggest that CA directly inhibits TLR4-MyD88-dependent signaling pathways and decreases the inflammatory response in adipocytes.
MeSH Terms
-
Adipocytes*
Adipokines
Chemokine CCL2
Inflammation
Interleukin-6
Myeloid Differentiation Factor 88
Obesity
Phosphotransferases
RNA, Messenger
TNF Receptor-Associated Factor 6
Toll-Like Receptor 4
Tumor Necrosis Factor-alpha
Up-Regulation
Adipokines
Chemokine CCL2
Interleukin-6
Myeloid Differentiation Factor 88
Phosphotransferases
RNA, Messenger
TNF Receptor-Associated Factor 6
Toll-Like Receptor 4
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Himes RW, Smith CW. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 2010; 24:731–739.2. Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araújo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007; 56:1986–1998.
Article3. Kim SJ, Choi Y, Choi YH, Park T. Obesity activates toll-like receptor-mediated proinflammatory signaling cascades in the adipose tissue of mice. J Nutr Biochem. 2012; 23:113–122.
Article4. Kopp A, Buechler C, Neumeier M, Weigert J, Aslanidis C, Schölmerich J, Schäffler A. Innate immunity and adipocyte function: ligand-specific activation of multiple Toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity (Silver Spring). 2009; 17:648–656.
Article5. Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, Kopp A, Schoelmerich J, Falk W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009; 126:233–245.
Article6. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007; 56:1761–1772.
Article7. Ibarra A, Cases J, Roller M, Chiralt-Boix A, Coussaert A, Ripoll C. Carnosic acid-rich rosemary (Rosmarinus officinalis L.) leaf extract limits weight gain and improves cholesterol levels and glycaemia in mice on a high-fat diet. Br J Nutr. 2011; 106:1182–1189.
Article8. Wang T, Takikawa Y, Satoh T, Yoshioka Y, Kosaka K, Tatemichi Y, Suzuki K. Carnosic acid prevents obesity and hepatic steatosis in ob/ob mice. Hepatol Res. 2011; 41:87–92.
Article9. Xiang Q, Liu Z, Wang Y, Xiao H, Wu W, Xiao C, Liu X. Carnosic acid attenuates lipopolysaccharide-induced liver injury in rats via fortifying cellular antioxidant defense system. Food Chem Toxicol. 2013; 53:1–9.
Article10. Gaya M, Repetto V, Toneatto J, Anesini C, Piwien-Pilipuk G, Moreno S. Antiadipogenic effect of carnosic acid, a natural compound present in Rosmarinus officinalis, is exerted through the C/EBPs and PPARγ pathways at the onset of the differentiation program. Biochim Biophys Acta. 2013; 1830:3796–3806.
Article11. Tsai CW, Liu KL, Lin YR, Kuo WC. The mechanisms of carnosic acid attenuates tumor necrosis factor-α-mediated inflammation and insulin resistance in 3T3-L1 adipocytes. Mol Nutr Food Res. 2014; 58:654–664.
Article12. Nagasaki H, Yoshimura T, Aoki N. Real-time monitoring of inflammation status in 3T3-L1 adipocytes possessing a secretory Gaussia luciferase gene under the control of nuclear factor-kappa B response element. Biochem Biophys Res Commun. 2012; 420:623–627.
Article13. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.
Article14. Park M, Han J, Lee CS, Soo BH, Lim KM, Ha H. Carnosic acid, a phenolic diterpene from rosemary, prevents UV-induced expression of matrix metalloproteinases in human skin fibroblasts and keratinocytes. Exp Dermatol. 2013; 22:336–341.
Article15. Meng P, Yoshida H, Matsumiya T, Imaizumi T, Tanji K, Xing F, Hayakari R, Dempoya J, Tatsuta T, Aizawa-Yashiro T, Mimura J, Kosaka K, Itoh K, Satoh K. Carnosic acid suppresses the production of amyloid-β 1-42 by inducing the metalloprotease gene TACE/ADAM17 in SH-SY5Y human neuroblastoma cells. Neurosci Res. 2013; 75:94–102.
Article16. O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007; 7:353–364.17. Sun W, Yang J. Molecular basis of lysophosphatidic acid-induced NF-κB activation. Cell Signal. 2010; 22:1799–1803.
Article18. Fresno M, Alvarez R, Cuesta N. Toll-like receptors, inflammation, metabolism and obesity. Arch Physiol Biochem. 2011; 117:151–164.
Article19. Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007; 132:2169–2180.
Article20. Anderson PD, Mehta NN, Wolfe ML, Hinkle CC, Pruscino L, Comiskey LL, Tabita-Martinez J, Sellers KF, Rickels MR, Ahima RS, Reilly MP. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab. 2007; 92:2272–2279.
Article21. Starr ME, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci. 2009; 64:723–730.
Article22. Tsai TH, Chuang LT, Lien TJ, Liing YR, Chen WY, Tsai PJ. Rosmarinus officinalis extract suppresses Propionibacterium acnes-induced inflammatory responses. J Med Food. 2013; 16:324–333.
Article23. Oh J, Yu T, Choi SJ, Yang Y, Baek HS, An SA, Kwon LK, Kim J, Rho HS, Shin SS, Choi WS, Hong S, Cho JY. Syk/Src pathway-targeted inhibition of skin inflammatory responses by carnosic acid. Mediators Inflamm. 2012; 2012:781375.
Article24. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002; 298:1911–1912.
Article25. Kopp A, Buechler C, Bala M, Neumeier M, Schölmerich J, Schäffler A. Toll-like receptor ligands cause proinflammatory and prodiabetic activation of adipocytes via phosphorylation of extracellular signal-regulated kinase and c-Jun N-terminal kinase but not interferon regulatory factor-3. Endocrinology. 2010; 151:1097–1108.
Article26. Barbarroja N, López-Pedrera R, Mayas MD, García-Fuentes E, Garrido-Sánchez L, Macías-González M, El Bekay R, Vidal-Puig A, Tinahones FJ. The obese healthy paradox: is inflammation the answer? Biochem J. 2010; 430:141–149.
Article27. Rasoolijazi H, Azad N, Joghataei MT, Kerdari M, Nikbakht F, Soleimani M. The protective role of carnosic acid against beta-amyloid toxicity in rats. ScientificWorldJournal. 2013; 2013:917082.
Article28. Rezaie T, McKercher SR, Kosaka K, Seki M, Wheeler L, Viswanath V, Chun T, Joshi R, Valencia M, Sasaki S, Tozawa T, Satoh T, Lipton SA. Protective effect of carnosic acid, a pro-electrophilic compound, in models of oxidative stress and light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2012; 53:7847–7854.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Carnosic Acid Inhibits Lipid Accumulation in 3T3-L1 Adipocytes Through Attenuation of Fatty Acid Desaturation
- Role of heat shock protein 70 in regulation of anti-inflammatory response to curcumin in 3T3-L1 adipocytes
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3