J Vet Sci.
2020 Jan;21(1):e6. 10.4142/jvs.2020.21.e6.
Comparison of the trometamol-balanced solution with two other crystalloid solutions for fluid resuscitation of a rat hemorrhagic model
- Affiliations
-
- 1Department and Graduate Institute of Veterinary Medicine, School of Veterinary Medicine, National Taiwan University, Taipei 10617, Taiwan. jacklee@ntu.edu.tw
- 2Cardiovascular Surgery, Department of Surgery, National Taiwan University Hospital, National Taiwan University, Taipei 10002, Taiwan. yschen1234@gmail.com
- 3Graduate Institute of Veterinary Clinical Sciences, School of Veterinary Medicine, National Taiwan University, Taipei 10672, Taiwan.
- 4Animal Cancer Treatment Center, National Taiwan University Veterinary Hospital, Taipei 10672, Taiwan.
- KMID: 2469021
- DOI: http://doi.org/10.4142/jvs.2020.21.e6
Abstract
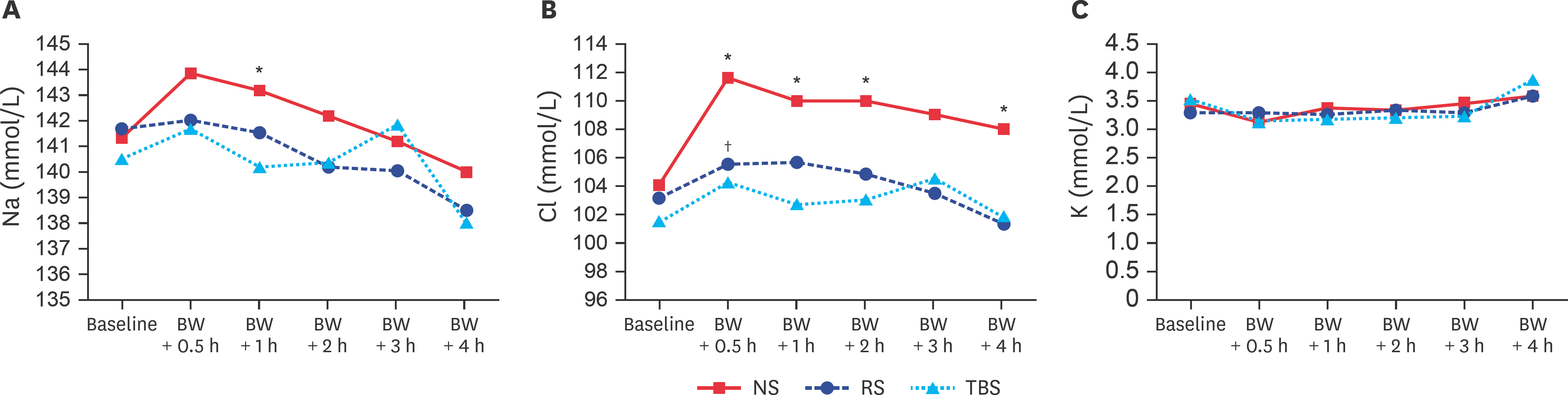
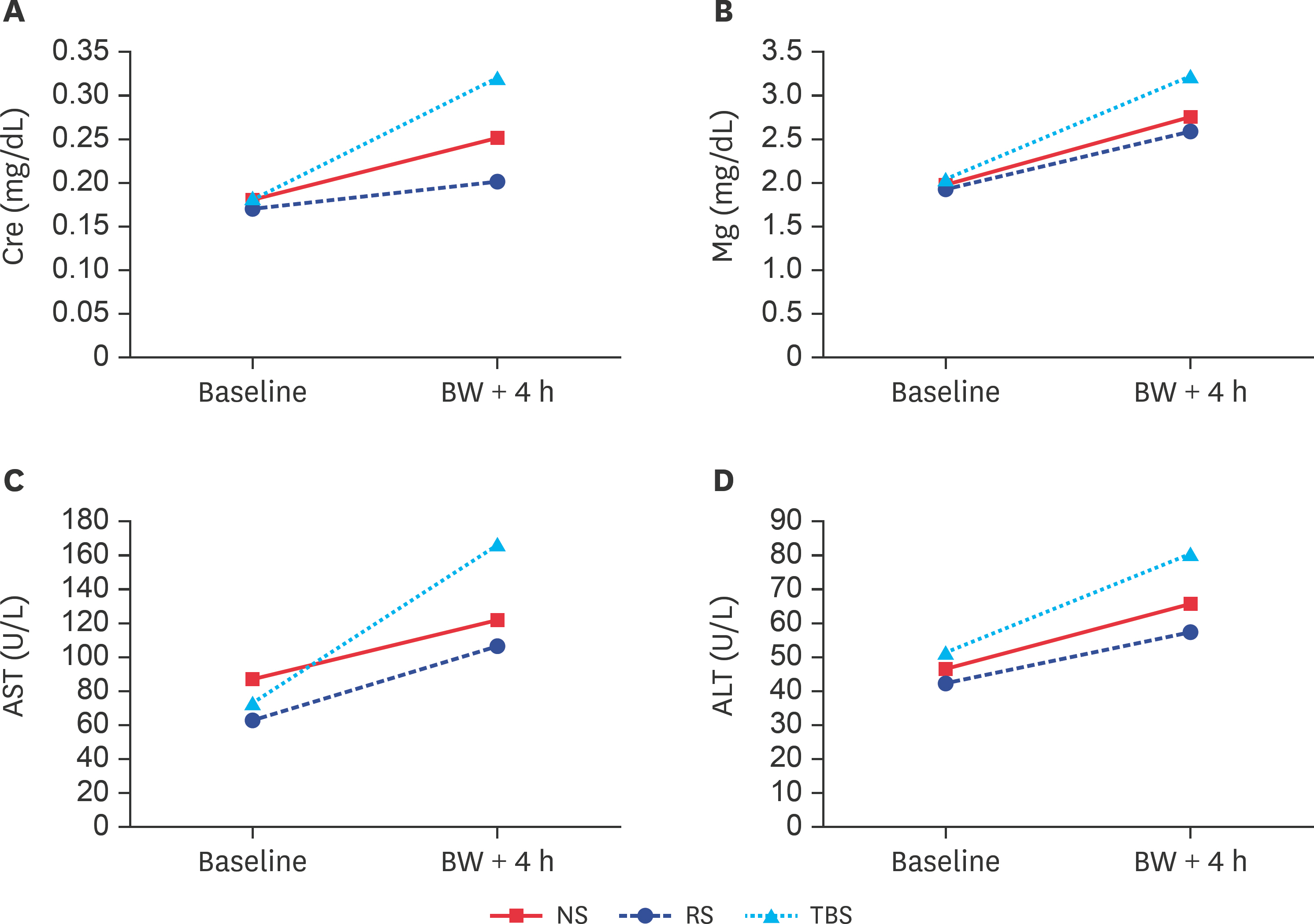
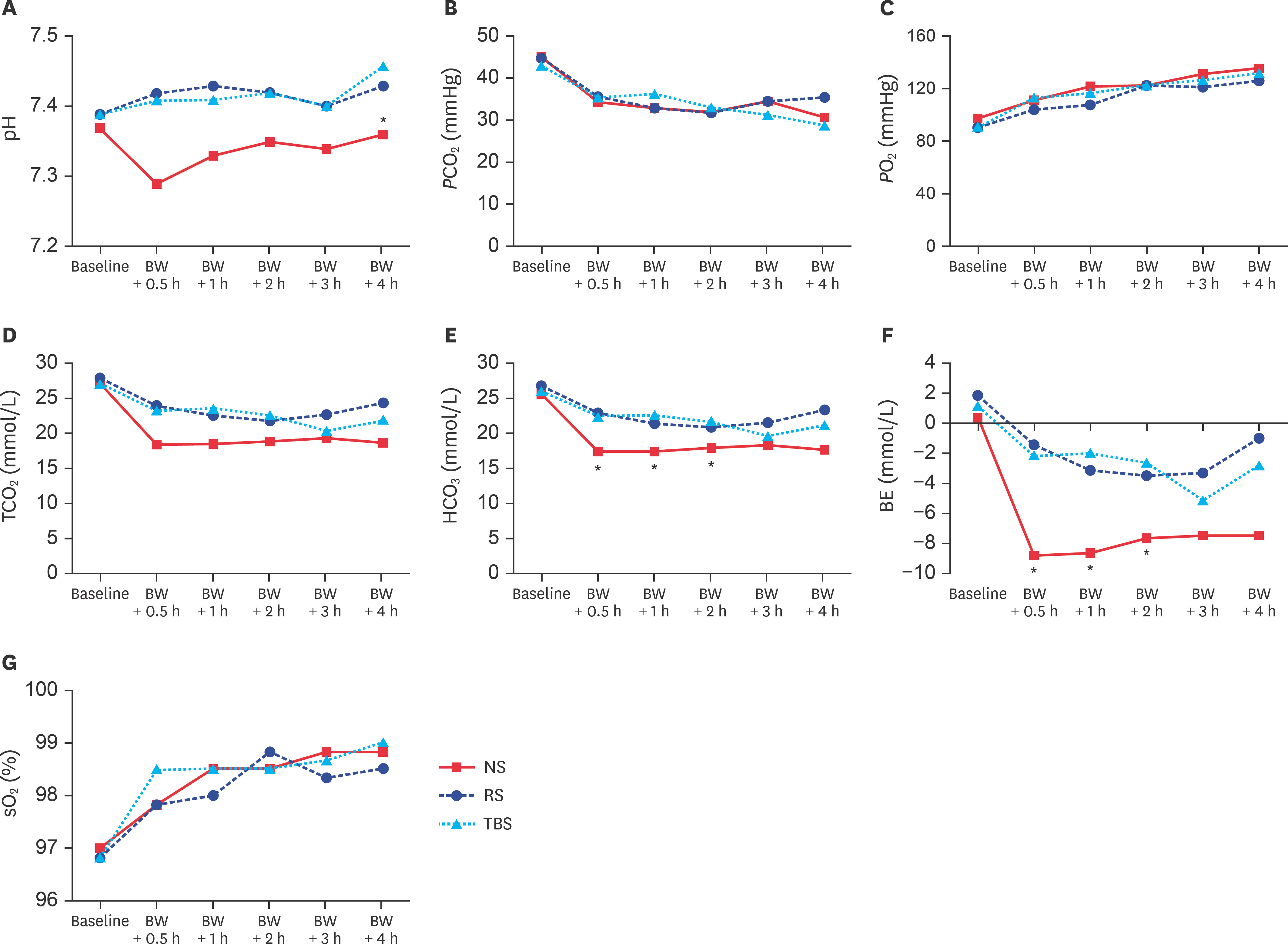
- Currently, the optimal resuscitation fluid remains debatable. Therefore, in the present study, we designed a trometamol-balanced solution (TBS) for use as a resuscitation fluid for hemorrhagic shock. Hemorrhagic shock was induced in 18 male Wistar-Kyoto rats, which were assigned to normal saline (NS), Ringer's solution (RS), and TBS groups. During the hemorrhagic state, their hemodynamic parameters were recorded using an Abbott i-STAT analyzer with the CG4+ cartridge (for pH, pressure of carbon dioxide, pressure of oxygen, total carbon dioxide, bicarbonate, base excess, oxygen saturation, and lactate), the CG6+ cartridge (for sodium, potassium, chloride, blood glucose, blood urea nitrogen, hematocrit, and hemoglobin), and enzyme-linked immunosorbent assay kits (calcium, magnesium, creatinine, aspartate aminotransferase, alanine aminotransferase, bilirubin, and albumin). Similar trends were found for the parameters of biochemistries, electrolytes, and blood gas, and they revealed no significant changes after blood withdrawal-induced hemorrhagic shock. However, the TBS group showed more effective ability to correct metabolic acidosis than the NS and RS groups. TBS was a feasible and safe resuscitation solution in this study and may be an alternative to NS and RS for resuscitation in hemorrhagic shock patients without liver damage.
MeSH Terms
-
Acidosis
Alanine Transaminase
Animals
Aspartate Aminotransferases
Bilirubin
Blood Glucose
Blood Urea Nitrogen
Carbon Dioxide
Creatinine
Electrolytes
Enzyme-Linked Immunosorbent Assay
Hematocrit
Hemodynamics
Humans
Hydrogen-Ion Concentration
Liver
Magnesium
Male
Oxygen
Potassium
Rats*
Resuscitation*
Shock, Hemorrhagic
Sodium
Alanine Transaminase
Aspartate Aminotransferases
Bilirubin
Blood Glucose
Carbon Dioxide
Creatinine
Electrolytes
Magnesium
Oxygen
Potassium
Sodium
Figure
Reference
-
References
1. Bonanno FG. Hemorrhagic shock: The “physiology approach”. J Emerg Trauma Shock. 2012; 5:285–295.
Article2. Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit Care. 2004; 8:373–381.3. Spinella PC, Holcomb JB. Resuscitation and transfusion principles for traumatic hemorrhagic shock. Blood Rev. 2009; 23:231–240.
Article4. Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013; 369:1243–1251.
Article5. Finfer S, Liu B, Taylor C, Bellomo R, Billot L, Cook D, Du B, McArthur C, Myburgh J, Investigators SA. SAFE TRIPS Investigators. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care. 2010; 14:R185.6. Lira A, Pinsky MR. Choices in fluid type and volume during resuscitation: impact on patient outcomes. Ann Intensive Care. 2014; 4:38.
Article7. Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2013; 2:CD000567.
Article8. Singer M. Management of fluid balance: a European perspective. Curr Opin Anaesthesiol. 2012; 25:96–101.9. Canabal JM, Kramer DJ. Management of sepsis in patients with liver failure. Curr Opin Crit Care. 2008; 14:189–197.
Article10. Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Selective microvascular endothelial cell dysfunction in the small intestine following resuscitated hemorrhagic shock. Shock. 1998; 10:417–422.
Article11. Hurt RT, Zakaria R, Matheson PJ, Cobb ME, Parker JR, Garrison RN. Hemorrhage-induced hepatic injury and hypoperfusion can be prevented by direct peritoneal resuscitation. J Gastrointest Surg. 2009; 13:587–594.
Article12. Zakaria R, Spain DA, Harris PD, Garrison RN. Resuscitation regimens for hemorrhagic shock must contain blood. Shock. 2002; 18:567–573.
Article13. Osthaus WA, Sievers J, Breymann T, Suempelmann R. Bicarbonate buffered ultrafiltration leads to a physiologic priming solution in pediatric cardiac surgery. Interact Cardiovasc Thorac Surg. 2008; 7:969–972.
Article14. Himpe D, Neels H, De Hert S, Van Cauwelaert P. Adding lactate to the prime solution during hypothermic cardiopulmonary bypass: a quantitative acid-base analysis. Br J Anaesth. 2003; 90:440–445.
Article15. Takkunen O, Salmenperä M, Heinonen J. Comparison of Ringer's acetate and lactate solutions as a prime for cardiopulmonary bypass. Ann Chir Gynaecol. 1985; 74:223–227.16. Nahas GG, Sutin KM, Fermon C, Streat S, Wiklund L, Wahlander S, Yellin P, Brasch H, Kanchuger M, Capan L, Manne J, Helwig H, Gaab M, Pfenninger E, Wetterberg T, Holmdahl M, Turndorf H. Guidelines for the treatment of acidaemia with THAM. Drugs. 1998; 55:191–224.
Article17. Kohut LK, Darwiche SS, Brumfield JM, Frank AM, Billiar TR. Fixed volume or fixed pressure: a murine model of hemorrhagic shock. J Vis Exp. 2011; 52:2068.
Article18. Bouchard JE, Mehta RL. Fluid balance issues in the critically ill patient. Contrib Nephrol. 2010; 164:69–78.
Article19. Krausz MM. Initial resuscitation of hemorrhagic shock. World J Emerg Surg. 2006; 1:14.20. Lee JW. Fluid and electrolyte disturbances in critically ill patients. Electrolyte Blood Press. 2010; 8:72–81.
Article21. Spaniol JR, Knight AR, Zebley JL, Anderson D, Pierce JD. Fluid resuscitation therapy for hemorrhagic shock. J Trauma Nurs. 2007; 14:152–160.
Article22. Wolf MB. Hyperglycemia-induced hyponatremia: reevaluation of the Na+ correction factor. J Crit Care. 2017; 42:54–58.23. Besunder JB, Smith PG. Toxic effects of electrolyte and trace mineral administration in the intensive care unit. Crit Care Clin. 1991; 7:659–693.
Article24. Drueke TB, Lacour B. Disorders of calcium, phosphate and magnesium metabolism. Feehally J, Floege J, Johnson RJ, editors. (eds.).Comprehensive Clinical Nephrology. 3rd ed.pp.p. 37–138. Mosby;Philadelphia: 2007.25. Zaloga GP. Hypocalcemia in critically ill patients. Crit Care Med. 1992; 20:251–262.
Article26. Zivin JR, Gooley T, Zager RA, Ryan MJ. Hypocalcemia: a pervasive metabolic abnormality in the critically ill. Am J Kidney Dis. 2001; 37:689–698.
Article27. Forsythe RM, Wessel CB, Billiar TR, Angus DC, Rosengart MR. Parenteral calcium for intensive care unit patients. Cochrane Database Syst Rev. 2008; 4:CD006163.
Article28. Collage RD, Howell GM, Zhang X, Stripay JL, Lee JS, Angus DC, Rosengart MR. Calcium supplementation during sepsis exacerbates organ failure and mortality via calcium/calmodulin-dependent protein kinase kinase signaling. Crit Care Med. 2013; 41:e352–e360.
Article29. Hirasawa H, Oda S, Nakamura M. Blood glucose control in patients with severe sepsis and septic shock. World J Gastroenterol. 2009; 15:4132–4136.
Article30. Losser MR, Damoisel C, Payen D. Bench-to-bedside review: glucose and stress conditions in the intensive care unit. Crit Care. 2010; 14:231.
Article31. Wernly B, Lichtenauer M, Hoppe UC, Jung C. Hyperglycemia in septic patients: an essential stress survival response in all, a robust marker for risk stratification in some, to be messed with in none. J Thorac Dis. 2016; 8:E621–E624.
Article32. Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012; 2:1303–1353.
Article33. Mayeur N, Minville V, Jaafar A, Allard J, Al Saati T, Guilbeau-Frugier C, Fourcade O, Girolami JP, Schaak S, Tack I. Morphologic and functional renal impact of acute kidney injury after prolonged hemorrhagic shock in mice. Crit Care Med. 2011; 39:2131–2138.
Article34. Wang L, Song J, Buggs J, Wei J, Wang S, Zhang J, Zhang G, Lu Y, Yip KP, Liu R. A new mouse model of hemorrhagic shock-induced acute kidney injury. Am J Physiol Renal Physiol. 2017; 312:F134–F142.
Article35. Koskinas J, Gomatos IP, Tiniakos DG, Memos N, Boutsikou M, Garatzioti A, Archimandritis A, Betrosian A. Liver histology in ICU patients dying from sepsis: a clinicopathological study. World J Gastroenterol. 2008; 14:1389–1393.
Article36. Soleimanpour H, Safari S, Rahmani F, Nejabatian A, Alavian SM. Hepatic shock differential diagnosis and risk factors: a review article. Hepat Mon. 2015; 15:e27063.
Article37. Akkose S, Ozgurer A, Bulut M, Koksal O, Ozdemír F, Ozguç H. Relationships between markers of inflammation, severity of injury, and clinical outcomes in hemorrhagic shock. Adv Ther. 2007; 24:955–962.
Article38. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013; 88:1127–1140.
Article39. Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985; 26:72–76.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Comparison of the Effects of Various Crystalloid Solutions on the Resuscitation in Rabbits with Acute Hemorrhagic Shock
- Fluid Management of Trauma Patients
- Fluid resuscitation in hemorrhagic shock model using 4% modified fluid gelatin(gelofusine) solution
- Crystalloid Resuscitation in Experimental Hemorrhagic Shock in Dogs
- Fluid therapy: classification and characteristics of intravenous fluids