Nat Prod Sci.
2019 Dec;25(4):341-347. 10.20307/nps.2019.25.4.341.
The Activation of PPAR-α and Wnt/β-catenin by Luffa cylindrica Supercritical Carbon Dioxide Extract
- Affiliations
-
- 1Division of Biomedicinal Chemistry and Cosmetics, Mokwon University, Daejeon 35349, Republic of Korea. bora0507@mokwon.ac.kr
- KMID: 2468068
- DOI: http://doi.org/10.20307/nps.2019.25.4.341
Abstract
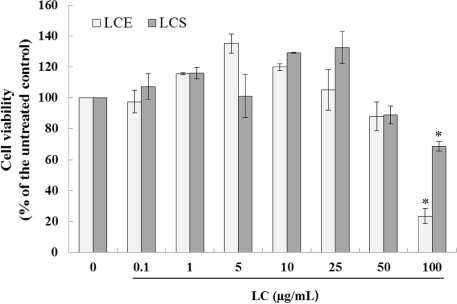
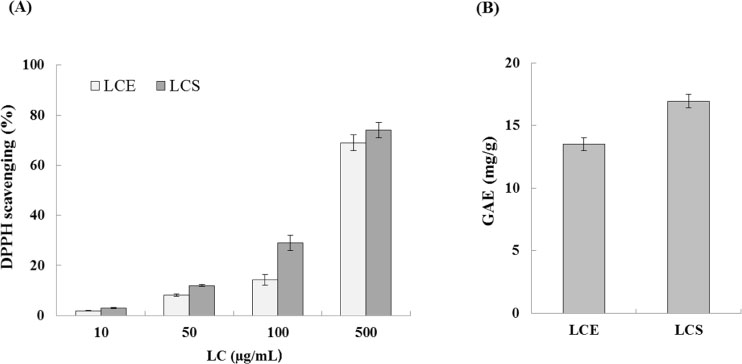
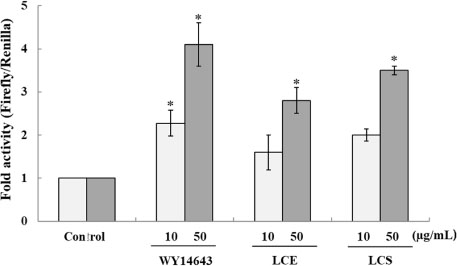
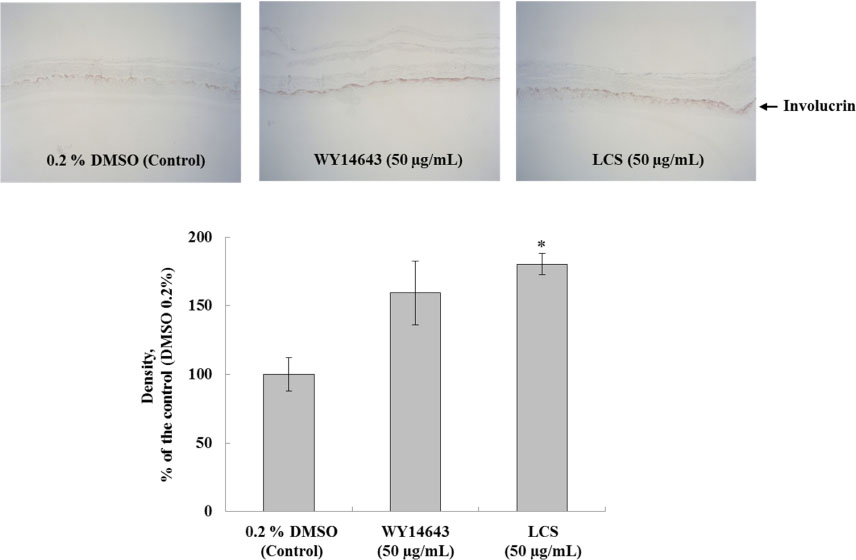
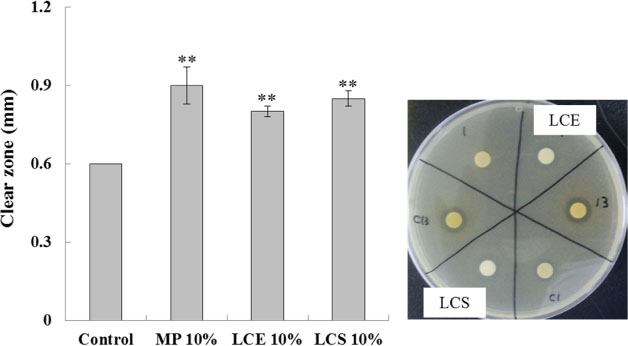
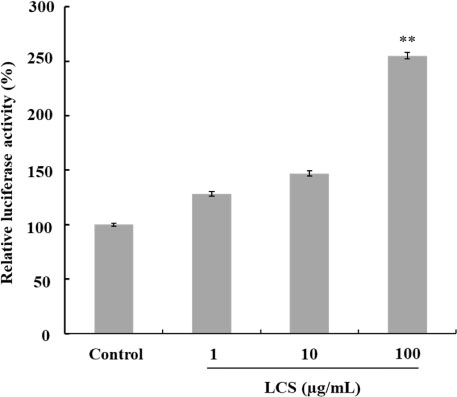
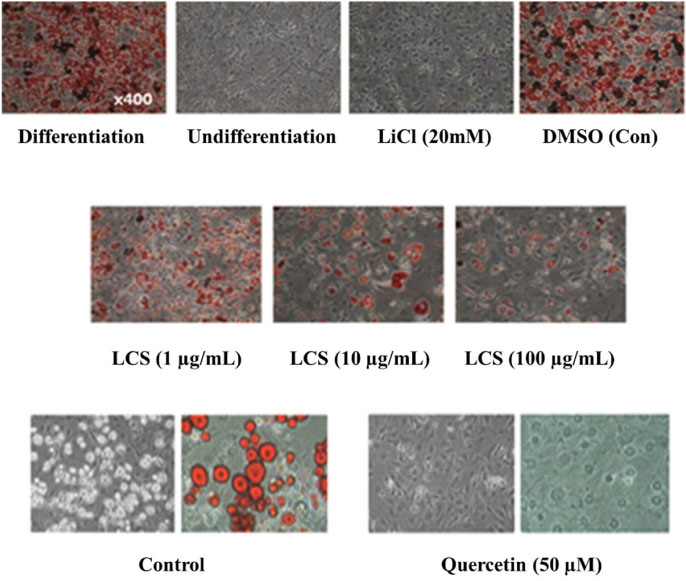
- Luffa cylindrica (LC) is a very fast-growing climber and its fruit have been considered as agricultural wastes. We conducted to check the comparative qualities of ethanol solvent extraction (LCE) and supercritical carbon dioxide extraction (LCS) of L. cylindrica fruit and seed. LCS had higher antioxidant and polyphenol contents than LCE. LCS were significantly increased peroxisome proliferator-activated receptor (PPAR)-a and involucrin expression as epidermal differentiation marker in 3D skin equivalent model. LCS also showed antimicrobial activity against Staphylococcus aureus, a causative bacteria in atopic dermatitis. In addition, LCS inhibited the adipocyte differentiation of 3T3-L1 cells. When treated with the extract at a concentration of 100 µg/mL, the Wnt/β-catenin pathway reporter luciferase activity of HEK 293-TOP cells was increased approximately by 2-folds compared to that of the untreated control group. These results indicate that L. cylindrica supercritical carbon dioxide extract may serve as a cosmeceutical for improving skin barrier function and the treatment of obesity.
Keyword
MeSH Terms
Figure
Reference
-
1. Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Plants. 2017; 6:42–65.2. Rivier M, Safonova I, Lebrun P, Griffiths CE, Ailhaud G, Michel S. J Invest Dermatol. 1998; 111:1116–1121.3. Tyśkiewicz K, Konkol M, Roj E. Molecules. 2018; 23:2625–2652.4. Komuves LG, Hanley K, Lefebvre AM, Man MQ, Ng DC, Bikle DD, Williams ML, Elias PM, Auwerx J, Feingold KR. J Invest Dermatol. 2000; 115:353–360.5. Liu J, Farmer SR. J Biol Chem. 2004; 279:45020–45027.6. Kennell JA, MacDougald OA. J Biol Chem. 2005; 280:24004–24010.7. Fruhbeck G, Gomez-Ambrosi J, Muruzabal FJ, Burrell MA. Am J Physiol Endocrinol Metab. 2001; 280:E827–E827.8. Heiser CB, Schilling EE. Biotropica. 1988; 20:185–191.9. Du Q, Xu Y, Li L, Zhao Y, Jerz G, Winterhalter P. J Agri Food Chem. 2006; 54:4186–4190.10. Kim B, Lee SM, Hwang T, Kim H. Korean J Food Preserv. 2013; 20:597–601.11. Lee SH, Kim B, Oh MJ, Yoon J, Kim HY, Lee KJ, Lee JD, Choi KY. Phytother Res. 2011; 25:1629–1635.12. Kim B, Choi YE, Kim HS. Phytother Res. 2014; 28:1359–1366.13. Kim B, Kim HS. Eur J Pharmacol. 2018; 832:25–32.14. Kim SH, Nam GW, Lee HK, Moon SJ, Chang IS. Arch Dermatol Res. 2006; 298:273–282.15. Sharma KV, Sisodia R. J Radiol Prot. 2009; 29:429–443.16. Dall'Acqua S, Cervellati R, Loi MC, Innocenti G. Food Chem. 2008; 106:745–749.17. Jensen JM, Folster-Holst R, Baranowsky A, Schunck M, Winoto-Morbach S, Neumann C, Schutze S, Proksch E. J Invest Dermatol. 2004; 122:1423–1431.18. Kim J, Kim BE, Ahn K, Leung DYM. Allergy Asthma Immunol Res. 2019; 11:593–603.19. Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Trends Endocrinol Metab. 2009; 20:16–24.20. Seo MJ, Lee YJ, Hwang JH, Kim KJ, Lee BY. J Nutr Biochem. 2015; 26:1308–1316.21. Kim MH, Park JS, Seo MS, Jung JW, Lee YS, Kang KS. Cell Prolif. 2010; 43:594–605.22. Ahn J, Lee H, Kim S, Ha T. Am J Physiol Cell Physiol. 2010; 298:C1510–C1516.23. Kim MH, Park JS, Seo MS, Jung JW, Lee YS, Kang KS. Cell Prolif. 2010; 43:594–605.24. Ahn J, Lee H, Kim S, Park J, Ha T. Biochem Biophys Res Commun. 2008; 373:545–549.25. Xue J, Zhu W, Song J, Jiao Y, Luo J, Yu C, Zhou J, Wu J, Chen M, Ding WQ, Cao J, Zhang S. Oncogene. 2018; 37:953–962.26. Vallee A, Lecarpentier Y. Front Neurosci. 2016; 10:459.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Natural Products Targeting Wnt/β-catenin Signaling Pathway
- Activation of the wnt/β-Catenin Signaling Pathway in Polymyositis, Dermatomyositis and Duchenne Muscular Dystrophy
- The crosstalk of Wnt/β-catenin signaling and p53 in acute kidney injury and chronic kidney disease
- Circular RNAs Regulate Cancer Onset and Progression via Wnt/β-Catenin Signaling Pathway
- β-Catenin expression is associated with cell invasiveness in pancreatic cancer