J Clin Neurol.
2016 Jul;12(3):351-360. 10.3988/jcn.2016.12.3.351.
Activation of the wnt/β-Catenin Signaling Pathway in Polymyositis, Dermatomyositis and Duchenne Muscular Dystrophy
- Affiliations
-
- 1Department of Neurology, Qilu Hospital of Shandong University, Jian, China. chuanzhuyan@163.com
- 2Department of Neurobiology, Yale University School of Medicine, New Haven, CT, USA.
- 3Key Laboratory for Experimental Teratology of the Ministry of Education, School of Medicine, Shandong University, Jian, China.
- 4Brain Science Research Institute, Shandong University, Jian, China.
- KMID: 2354124
- DOI: http://doi.org/10.3988/jcn.2016.12.3.351
Abstract
- BACKGROUND AND PURPOSE
The wnt/β-catenin signaling pathway plays a critical role in embryonic development and adult-tissue homeostasis. Recent investigations implicate the importance of wnt/β-catenin signaling in normal wound healing and its sustained activation being associated with fibrogenesis. We investigated the immunolocalization and activation of wnt/β-catenin in polymyositis (PM), dermatomyositis (DM), and Duchenne muscular dystrophy (DMD).
METHODS
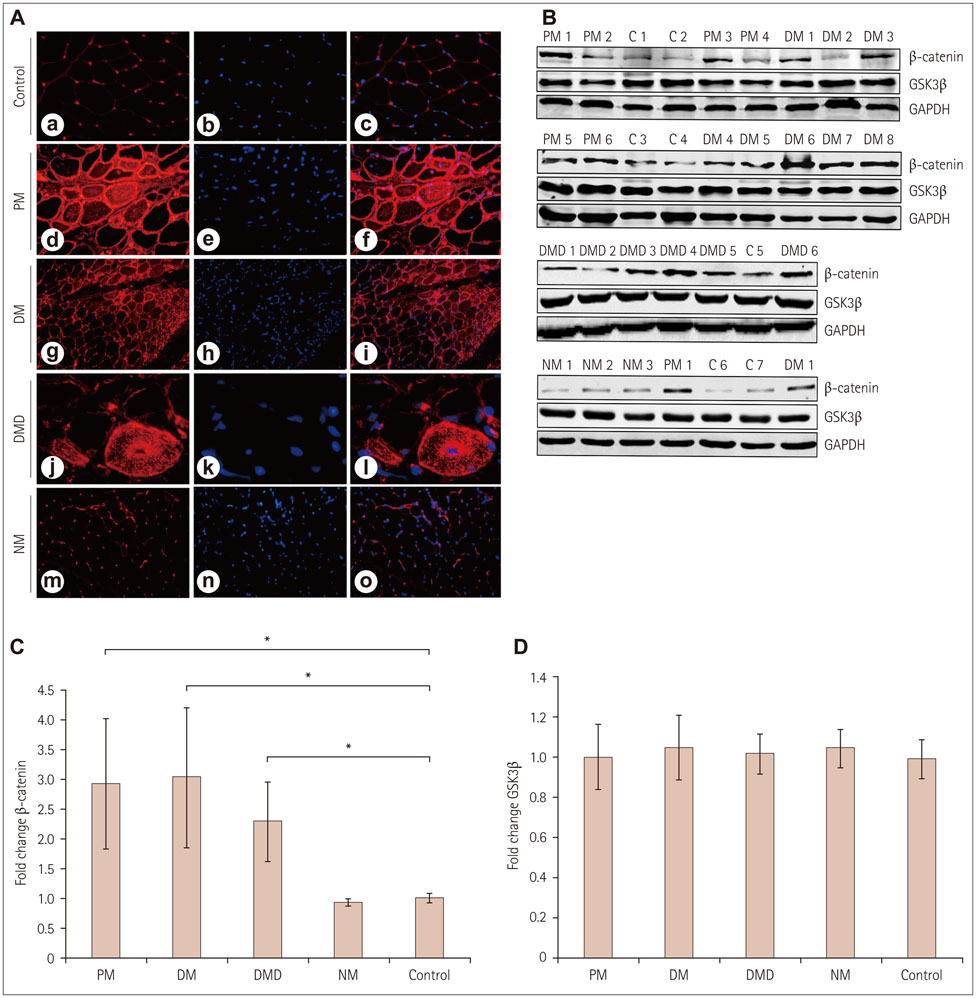
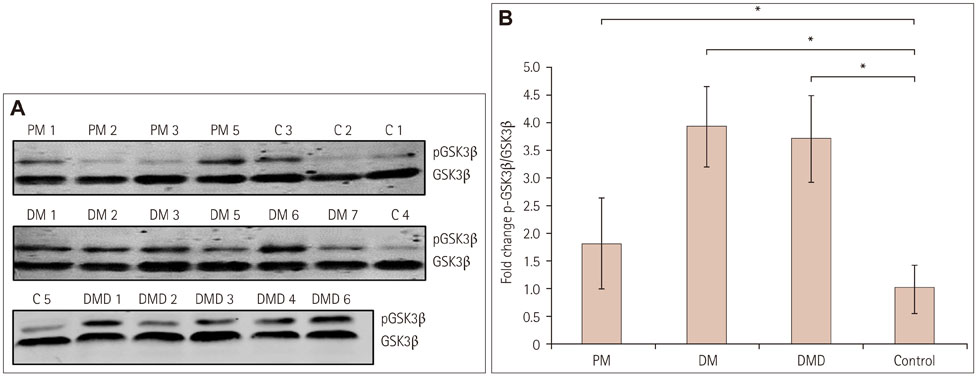
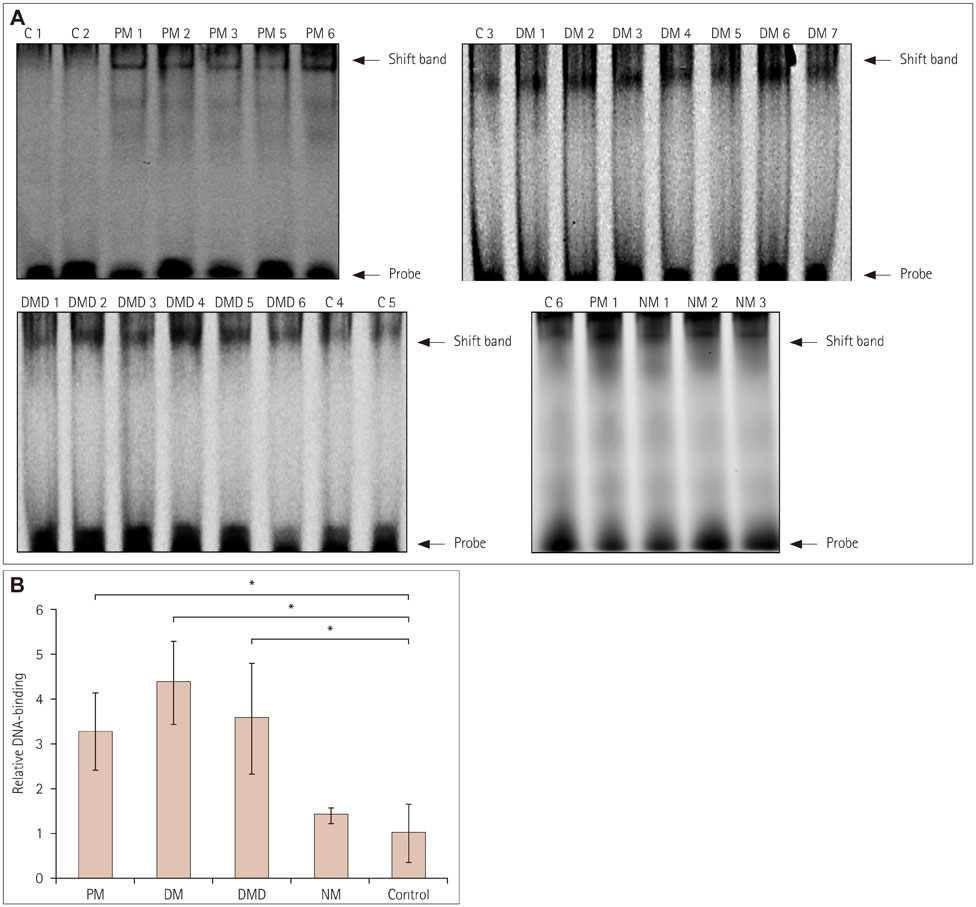
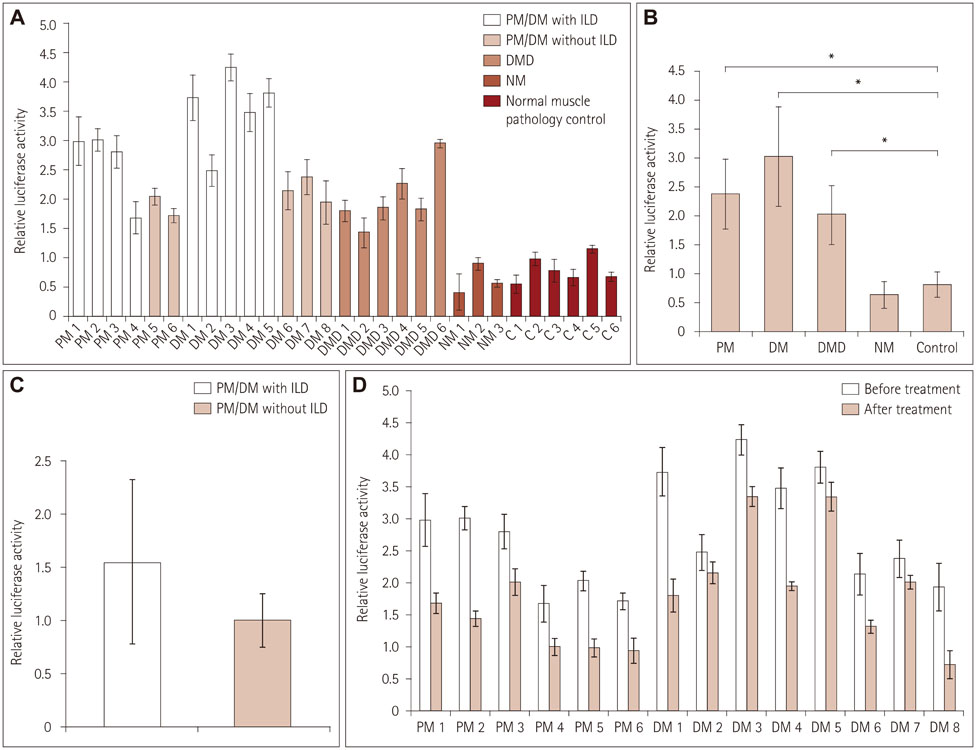
Immunofluorescence staining and Western blot analysis of β-catenin were performed in muscle specimens from 6 PM, 8 DM, and 6 DMD subjects. The β-catenin/Tcf4 DNA-binding activity in muscle was studied using an electrophoretic mobility shift assay (EMSA), and serum wnt/β-catenin/Tcf transcriptional activity was measured using a luciferase reporter gene assay.
RESULTS
Immunoreactivity for β-catenin was found in the cytoplasm and nuclei of muscle fibers in PM, DM, and DMD. The protein level of β-catenin was elevated, and EMSA analysis confirmed the activation of wnt/β-catenin signaling. The transcriptional activities of β-catenin/Tcf in the circulation were increased in patients with PM, DM, and DMD, especially in those with interstitial lung disease, and these transcriptional activities decreased when PM or DM patients exhibited obvious clinical improvements.
CONCLUSIONS
Our findings indicate that wnt/β-catenin signaling is activated in PM, DM, and DMD. Its activation in muscle tissue and the circulation may play a role in modulating muscle regeneration and be at least partly involved in the process of muscle and pulmonary fibrosis.
MeSH Terms
-
Blotting, Western
Cytoplasm
Dermatomyositis*
Electrophoretic Mobility Shift Assay
Embryonic Development
Female
Fluorescent Antibody Technique
Genes, Reporter
Homeostasis
Humans
Luciferases
Lung Diseases, Interstitial
Muscular Dystrophy, Duchenne*
Polymyositis*
Pregnancy
Pulmonary Fibrosis
Regeneration
Wound Healing
Luciferases
Figure
Reference
-
1. Dalakas MC. Autoimmune inflammatory myopathies. Handb Clin Neurol. 2007; 86:273–301.
Article2. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003; 362:971–982.
Article3. Kalluri M, Oddis CV. Pulmonary manifestations of the idiopathic inflammatory myopathies. Clin Chest Med. 2010; 31:501–512.
Article4. Monici MC, Aguennouz M, Mazzeo A, Messina C, Vita G. Activation of nuclear factor-kappaB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology. 2003; 60:993–997.
Article5. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012; 149:1192–1205.
Article6. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009; 17:9–26.7. Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006; 127:469–480.8. Lim JC, Kania KD, Wijesuriya H, Chawla S, Sethi JK, Pulaski L, et al. Activation of beta-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells. J Neurochem. 2008; 106:1855–1865.9. Petropoulos H, Skerjanc IS. Beta-catenin is essential and sufficient for skeletal myogenesis in P19 cells. J Biol Chem. 2002; 277:15393–15399.
Article10. Fujimaki S, Hidaka R, Asashima M, Takemasa T, Kuwabara T. Wnt protein-mediated satellite cell conversion in adult and aged mice following voluntary wheel running. J Biol Chem. 2014; 289:7399–7412.
Article11. Tanaka S, Terada K, Nohno T. Canonical Wnt signaling is involved in switching from cell proliferation to myogenic differentiation of mouse myoblast cells. J Mol Signal. 2011; 6:12.
Article12. Chilosi M, Poletti V, Zamò A, Lestani M, Montagna L, Piccoli P, et al. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003; 162:1495–1502.
Article13. He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009; 20:765–776.14. Sato M. Upregulation of the Wnt/beta-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm Venereol. 2006; 86:300–307.
Article15. Beyer C, Schramm A, Akhmetshina A, Dees C, Kireva T, Gelse K, et al. β-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis. 2012; 71:761–767.
Article16. Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007; 317:807–810.
Article17. Lévy L, Wei Y, Labalette C, Wu Y, Renard CA, Buendia MA, et al. Acetylation of beta-catenin by p300 regulates beta-catenin-Tcf4 interaction. Mol Cell Biol. 2004; 24:3404–3414.18. Tews DS. Tumour necrosis factor-mediated cell death pathways do not contribute to muscle fibre death in dystrophinopathies. Acta Neuropathol. 2005; 109:217–225.
Article19. Ghosh S, Paul A, Sen E. Tumor necrosis factor α-induced hypoxiainducible factor 1α-β-catenin axis regulates major histocompatibility complex class I gene activation through chromatin remodeling. Mol Cell Biol. 2013; 33:2718–2731.
Article20. Yang CC, Askanas V, Engel WK, Alvarez RB. Immunolocalization of transcription factor NF-kappaB in inclusion-body myositis muscle and at normal human neuromuscular junctions. Neurosci Lett. 1998; 254:77–80.
Article21. Liu YT, Shang D, Akatsuka S, Ohara H, Dutta KK, Mizushima K, et al. Chronic oxidative stress causes amplification and overexpression of ptprz1 protein tyrosine phosphatase to activate beta-catenin pathway. Am J Pathol. 2007; 171:1978–1988.
Article22. Banerji CR, Knopp P, Moyle LA, Severini S, Orrell RW, Teschendorff AE, et al. β-Catenin is central to DUX4-driven network rewiring in facioscapulohumeral muscular dystrophy. J R Soc Interface. 2015; 12:20140797.23. Abu-Baker A, Laganiere J, Gaudet R, Rochefort D, Brais B, Neri C, et al. Lithium chloride attenuates cell death in oculopharyngeal muscular dystrophy by perturbing Wnt/β-catenin pathway. Cell Death Dis. 2013; 4:e821.
Article24. Markiewicz E, Tilgner K, Barker N, van de Wetering M, Clevers H, Dorobek M, et al. The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus. EMBO J. 2006; 25:3275–3285.
Article25. Trensz F, Haroun S, Cloutier A, Richter MV, Grenier G. A muscle resident cell population promotes fibrosis in hindlimb skeletal muscles of mdx mice through the Wnt canonical pathway. Am J Physiol Cell Physiol. 2010; 299:C939–C947.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Natural Products Targeting Wnt/β-catenin Signaling Pathway
- Circular RNAs Regulate Cancer Onset and Progression via Wnt/β-Catenin Signaling Pathway
- Suppression of β-catenin Signaling Pathway in Human Prostate Cancer PC3 Cells by Delphinidin
- Regulation of Osteoblast Metabolism by Wnt Signaling
- Evaluation of expression of the Wnt signaling components in canine mammary tumors via RT² Profiler PCR Array and immunochemistry assays