Yonsei Med J.
2019 Dec;60(12):1187-1194. 10.3349/ymj.2019.60.12.1187.
MicroRNA-138 Suppresses Adipogenic Differentiation in Human Adipose Tissue-Derived Mesenchymal Stem Cells by Targeting Lipoprotein Lipase
- Affiliations
-
- 1Department of Burn Plastic Surgery, the Yuhuangding Hospital of Yantai, Yantai, China. zydmbp@163.com
- KMID: 2463798
- DOI: http://doi.org/10.3349/ymj.2019.60.12.1187
Abstract
- PURPOSE
Adipogenic differentiation of adipose tissue-derived mesenchymal stem cells (AMSCs) is critical to many disease-related disorders, such as obesity and diabetes. Studies have demonstrated that miRNA-138 (miR-138) is closely involved in adipogenesis. However, the mechanisms affected by miR-138 remain unclear. This work aimed to investigate interactions between miR-138 and lipoprotein lipase (LPL), a key lipogenic enzyme, in AMSCs.
MATERIALS AND METHODS
Human AMSCs (hAMSCs) isolated from human abdomen tissue were subjected to adipogenic differentiation medium. Quantitative real-time polymerase chain reaction and Western blot assay were applied to measure the expressions of miR-138, LPL, and the two adipogenic transcription factors cytidine-cytidine-adenosine-adenosine-thymidine enhancer binding protein alpha (C/EBPα) and peroxisome proliferator-activated receptor gamma (PPARγ). The relationship between miR-138 and LPL was predicted utilizing the miRTarBase database and validated by dual luciferase reporter assay.
RESULTS
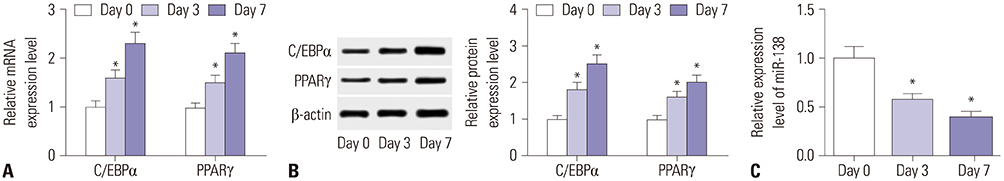
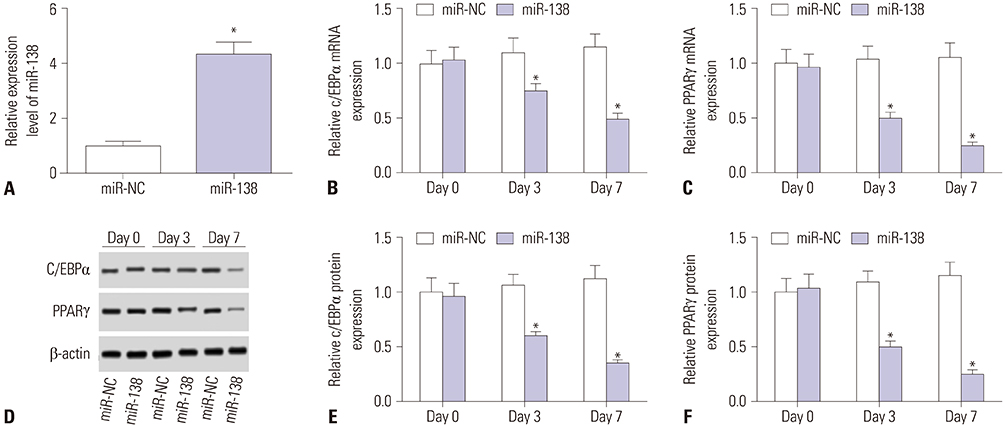
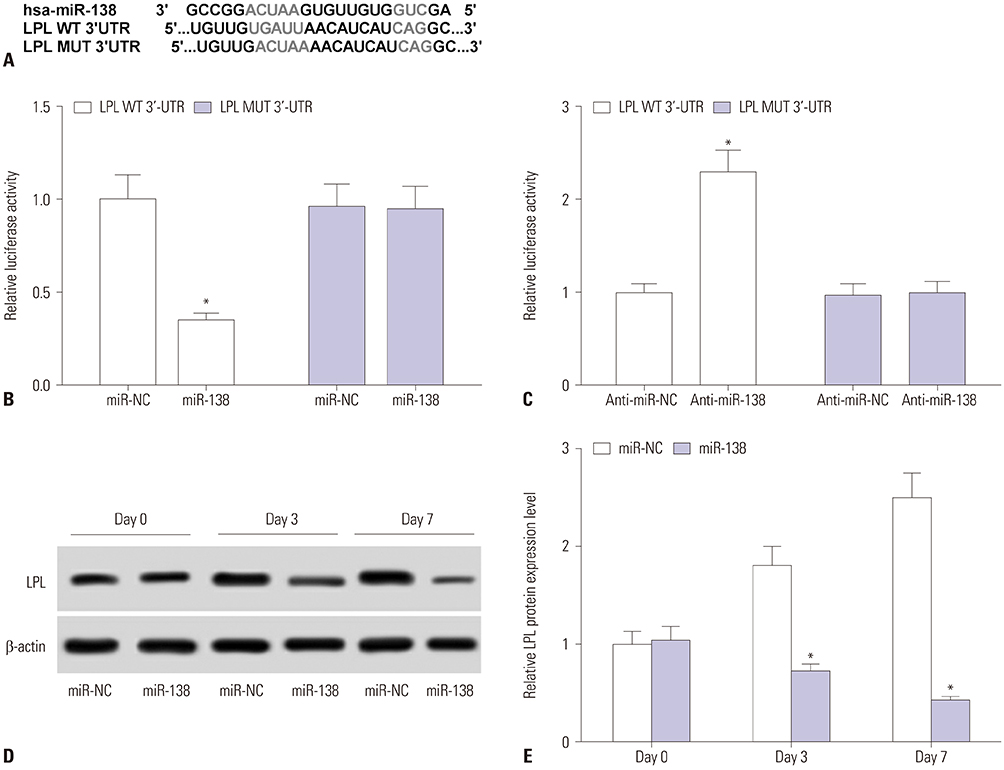
Showing increases in C/EBPα and PPARγ expression levels, hAMSCs were induced into adipogenic differentiation. During adipogenesis of hAMSCs, miR-138 expression was significantly downregulated. Overexpression of miR-138 by transfection inhibited hAMSCs adipogenic differentiation in vitro. Mechanically, LPL was a target of miR-138. LPL expression was upregulated during adipogenesis of hAMSCs, and this upregulation was reversed by miR-138 overexpression. Functionally, silencing of LPL by transfection exerted similar inhibition of the expressions of C/EBPα and PPARγ. Meanwhile, LPL ectopic expression was able to partly abolish the suppressive effect of miR-138 overexpression on adipogenic differentiation of hAMSCs.
CONCLUSION
Upregulation of miR-138 inhibits adipogenic differentiation of hAMSCs by directly downregulating LPL.
MeSH Terms
-
Abdomen
Adipogenesis
Blotting, Western
Carrier Proteins
Ectopic Gene Expression
Humans*
In Vitro Techniques
Lipoprotein Lipase*
Lipoproteins*
Luciferases
Mesenchymal Stromal Cells*
Obesity
PPAR gamma
Real-Time Polymerase Chain Reaction
Transcription Factors
Transfection
Up-Regulation
Carrier Proteins
Lipoprotein Lipase
Lipoproteins
Luciferases
PPAR gamma
Transcription Factors
Figure
Reference
-
1. Fitzsimmons REB, Mazurek MS, Soos A, Simmons CA. Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int. 2018; 2018:8031718.
Article2. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.
Article3. Moreira A, Kahlenberg S, Hornsby P. Therapeutic potential of mesenchymal stem cells for diabetes. J Mol Endocrinol. 2017; 59:R109–R120.
Article4. Lee JE, Schmidt H, Lai B, Ge K. Transcriptional and epigenomic regulation of adipogenesis. Mol Cell Biol. 2019; 39:e00601-18.
Article5. Yang HJ, Kim KJ, Kim MK, Lee SJ, Ryu YH, Seo BF, et al. The stem cell potential and multipotency of human adipose tissue-derived stem cells vary by cell donor and are different from those of other types of stem cells. Cells Tissues Organs. 2014; 199:373–383.
Article6. Mota de Sá P, Richard AJ, Hang H, Stephens JM. Transcriptional regulation of adipogenesis. Compr Physiol. 2017; 7:635–674.7. Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006; 7:885–896.
Article8. Almalki SG, Agrawal DK. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation. 2016; 92:41–51.
Article9. Hamam D, Ali D, Kassem M, Aldahmash A, Alajez NM. microRNAs as regulators of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2015; 24:417–425.
Article10. Ortega FJ, Moreno-Navarrete JM, Pardo G, Sabater M, Hummel M, Ferrer A, et al. MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS One. 2010; 5:e9022.
Article11. Skårn M, Namløs HM, Noordhuis P, Wang MY, Meza-Zepeda LA, Myklebost O. Adipocyte differentiation of human bone marrow-derived stromal cells is modulated by microRNA-155, microRNA-221, and microRNA-222. Stem Cells Dev. 2012; 21:873–883.
Article12. Hu X, Tang J, Hu X, Bao P, Pan J, Chen Z, et al. MiR-27b impairs adipocyte differentiation of human adipose tissue-derived mesenchymal stem cells by targeting LPL. Cell Physiol Biochem. 2018; 47:545–555.
Article13. Goff LA, Boucher S, Ricupero CL, Fenstermacher S, Swerdel M, Chase LG, et al. Differentiating human multipotent mesenchymal stromal cells regulate microRNAs: prediction of microRNA regulation by PDGF during osteogenesis. Exp Hematol. 2008; 36:1354–1369.
Article14. Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, et al. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem. 2004; 279:52361–52365.
Article15. Yu Y, Chen Y, Zhang X, Lu X, Hong J, Guo X, et al. Knockdown of lncRNA KCNQ1OT1 suppresses the adipogenic and osteogenic differentiation of tendon stem cell via downregulating miR-138 target genes PPARγ and RUNX2. Cell Cycle. 2018; 17:2374–2385.
Article16. Yang Z, Bian C, Zhou H, Huang S, Wang S, Liao L, et al. MicroRNA hsa-miR-138 inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells through adenovirus EID-1. Stem Cells Dev. 2011; 20:259–267.
Article17. Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab. 2009; 297:E271–E288.
Article18. Bouvy-Liivrand M, Heinäniemi M, John E, Schneider JG, Sauter T, Sinkkonen L. Combinatorial regulation of lipoprotein lipase by microRNAs during mouse adipogenesis. RNA Biol. 2014; 11:76–91.
Article19. Kim D, Kim J, Hyun H, Kim K, Roh S. A nanoscale ridge/groove pattern arrayed surface enhances adipogenic differentiation of human supernumerary tooth-derived dental pulp stem cells in vitro. Arch Oral Biol. 2014; 59:765–774.
Article20. Kim Y, Kim H, Cho H, Bae Y, Suh K, Jung J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem. 2007; 20:867–876.
Article21. Nardelli C, Granata I, Iaffaldano L, D'Argenio V, Del Monaco V, Maruotti GM, et al. miR-138/miR-222 overexpression characterizes the miRNome of amniotic mesenchymal stem cells in obesity. Stem Cells Dev. 2017; 26:4–14.
Article22. Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C, Ailhaud G, et al. microRNA miR-27b impairs human adipocyte differentiation and targets PPARgamma. Biochem Biophys Res Commun. 2009; 390:247–251.
Article23. Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim MM, Srikantan S, et al. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol. 2011; 31:626–638.
Article24. Sha H, Sun S, Francisco AB, Ehrhardt N, Xue Z, Liu L, et al. The ER-associated degradation adaptor protein Sel1L regulates LPL secretion and lipid metabolism. Cell Metab. 2014; 20:458–470.
Article25. Takagi A, Ikeda Y. [Genetic diagnosis on hypertriglyceridemia-analysis for LPL gene mutations]. Nihon Rinsho. 2013; 71:1569–1576.26. Martin PJ, Haren N, Ghali O, Clabaut A, Chauveau C, Hardouin P, et al. Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs). BMC Cell Biol. 2015; 16:10.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dlx3 and Dlx5 Inhibit Adipogenic Differentiation of Human Dental Pulp Stem Cells
- Characterization and differentiation into adipocytes of mesenchymal stem cells (MSCs) from human adipose tissue and amniotic fluid
- Comparison with human amniotic membrane- and adipose tissue-derived mesenchymal stem cells
- Adipogenesis of Sprague Dawely rats mesenchymal stem cells: a morphological, immunophenotyping and gene expression follow-up study
- Adipose-derived stem cells: characterization and clinical application