Clin Orthop Surg.
2019 Sep;11(3):361-368. 10.4055/cios.2019.11.3.361.
Platelet-Rich Plasma Pretreatment on Grit-Blasted Titanium Alloy for Enhanced Osteogenic Differentiation of Human Adipose-Derived Stem Cells
- Affiliations
-
- 1Department of Orthopedic Surgery, Seoul National University College of Medicine, Seoul, Korea. jyos@snu.ac.kr
- KMID: 2462567
- DOI: http://doi.org/10.4055/cios.2019.11.3.361
Abstract
- BACKGROUND
Adequate bone formation around titanium alloy implants is integral to successful implantation surgery. Stem cell-coated implants may accelerate peri-implant bone formation. This study investigates the effect of platelet-rich plasma (PRP) pretreatment on a titanium-alloy surface in terms of proliferation and osteogenic differentiation of human adipose-derived stem cells (hADSCs).
METHODS
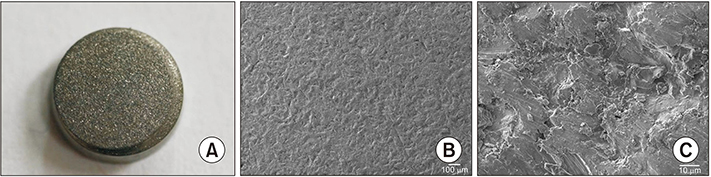
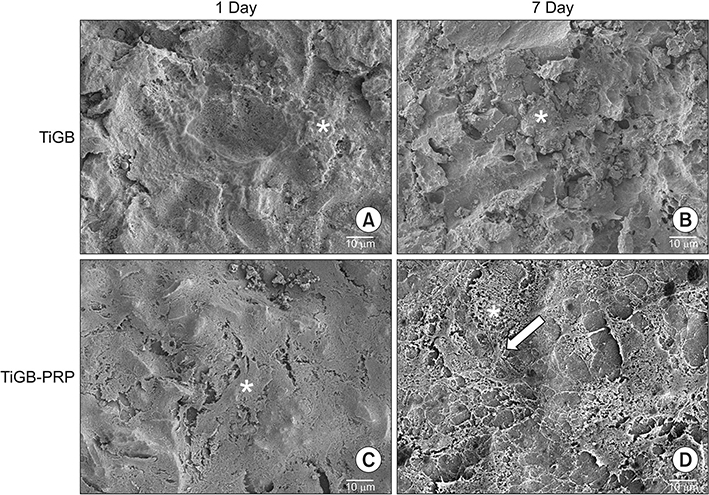
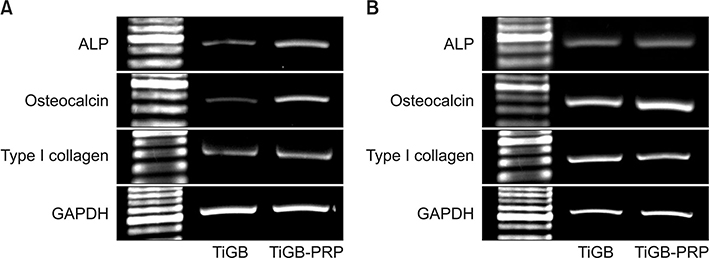
Allogenic leukocyte-depleted PRP was obtained from blood supernatants. The hADSCs were isolated from thigh subcutaneous fat tissue. Grit-blasted titanium plugs were used in two different groups. In one group, 200 µL of PRP was added to the grit-blasted titanium plugs. The hADSCs were seeded in two groups: grit-blasted titanium plugs with or without PRP. The number of hADSCs was measured after 4 hours, 3 days, and 7 days of culture using Cell Counting Kit-8. Osteogenesis of hADSCs was measured by using an alkaline phosphatase activity assay on days 7 and 14, and a calcium assay on days 14 and 21. Osteogenic gene expression was measured by using reverse transcription polymerase chain reaction analysis of alkaline phosphatase, osteocalcin, and type I collagen mRNA. The microscopic morphology of grit-blasted titanium plugs with or without PRP was examined with a field-emission scanning electron microscope using a JSM-7401F apparatus on days 1 and 7.
RESULTS
Proliferation and osteogenic differentiation of hADSCs were found to be significantly higher on the grit-blasted titanium alloy preprocessed with PRP than the same alloy without pretreatment. Furthermore, a structural fibrillar mesh developed compactly on the grit-blasted titanium alloy with the PRP pretreatment.
CONCLUSIONS
Our results demonstrate that a hADSC-based approach can be used for tissue-engineered peri-implant bone formation and that PRP pretreatment on the grit-blasted titanium alloy can improve proliferation and osteogenic differentiation of hADSCs.
Keyword
MeSH Terms
-
Alkaline Phosphatase
Alloys*
Calcium
Cell Count
Collagen Type I
Gene Expression
Humans*
Osteocalcin
Osteogenesis
Platelet-Rich Plasma*
Polymerase Chain Reaction
Reverse Transcription
RNA, Messenger
Stem Cells*
Subcutaneous Fat
Thigh
Titanium*
Alkaline Phosphatase
Alloys
Calcium
Collagen Type I
Osteocalcin
RNA, Messenger
Titanium
Figure
Reference
-
1. Han SK, Chang YJ, Kim YS, Lee JY, Lim YW. Effect of surface modification on biomechanical properties of titanium alloy Ti6Al4V. Tissue Eng Regen Med. 2010; 7(3):338–343.2. Malec K, Goralska J, Hubalewska-Mazgaj M, et al. Effects of nanoporous anodic titanium oxide on human adipose derived stem cells. Int J Nanomedicine. 2016; 11:5349–5360.
Article3. Lee MH, Oh NS, Lee SW, Kang JH, Lee SC, Leesungbok R. Enhancement of dynamic wettability, cell adhesion, and alkaline phosphatase activity of primary cells on titanium substrata with combined surface topographies of microgrooves and acid-etched roughness. Tissue Eng Regen Med. 2010; 7(5):501–512.4. Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells. 2007; 25(4):818–827.
Article5. Hempel U, Muller K, Preissler C, et al. Human bone marrow stromal cells: a reliable, challenging tool for in vitro osteogenesis and bone tissue engineering approaches. Stem Cells Int. 2016; 2016:7842191.6. Nae S, Bordeianu I, Stancioiu AT, Antohi N. Human adipose-derived stem cells: definition, isolation, tissue-engineering applications. Rom J Morphol Embryol. 2013; 54(4):919–924.7. Shin SH, Yoo JJ, Kim HN, Nam J, Kim HJ. Enhanced cellular responses of human bone marrow stromal cells cultured on pretreated surface with allogenic platelet-rich plasma. Connect Tissue Res. 2012; 53(4):318–326.
Article8. D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999; 14(7):1115–1122.9. Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev. 2001; 122(7):713–734.
Article10. Gir P, Oni G, Brown SA, Mojallal A, Rohrich RJ. Human adipose stem cells: current clinical applications. Plast Reconstr Surg. 2012; 129(6):1277–1290.11. Mehta S, Watson JT. Platelet rich concentrate: basic science and current clinical applications. J Orthop Trauma. 2008; 22(6):432–438.
Article12. van den Dolder J, Mooren R, Vloon AP, Stoelinga PJ, Jansen JA. Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng. 2006; 12(11):3067–3073.
Article13. Song HR, Bae JH, Park JH, et al. The effect of platelet rich plasma on osteogenesis in a long bone segmental defect : is the platelet rich plasma effective for bone reconstruction? Tissue Eng Regen Med. 2010; 7(4):395–400.14. Lee JH, Nam J, Nam KW, Kim HJ, Yoo JJ. Pre-treatment of titanium alloy with platelet-rich plasma enhances human osteoblast responses. Tissue Eng Regen Med. 2016; 13(4):335–342.
Article15. Zanicotti DG, Duncan WJ, Seymour GJ, Coates DE. Effect of titanium surfaces on the osteogenic differentiation of human adipose-derived stem cells. Int J Oral Maxillofac Implants. 2018; 33(3):e77–e87.
Article16. Gastaldi G, Asti A, Scaffino MF, et al. Human adipose-derived stem cells (hASCs) proliferate and differentiate in osteoblast-like cells on trabecular titanium scaffolds. J Biomed Mater Res A. 2010; 94(3):790–799.
Article17. Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008; 129(3):163–173.
Article18. Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006; 99(5):1285–1297.
Article19. Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006; 24(5):1294–1301.
Article20. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011; 39(10):2135–2140.
Article21. Sanchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003; 18(1):93–103.22. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010; 303(2):144–149.
Article23. Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants: a histomorphometric study in miniature pigs. J Biomed Mater Res. 1991; 25(7):889–902.
Article24. Lakstein D, Kopelovitch W, Barkay Z, Bahaa M, Hendel D, Eliaz N. Enhanced osseointegration of grit-blasted, NaOH-treated and electrochemically hydroxyapatite-coated Ti-6Al-4V implants in rabbits. Acta Biomater. 2009; 5(6):2258–2269.
Article25. Wu Y, Zitelli JP, TenHuisen KS, Yu X, Libera MR. Differential response of Staphylococci and osteoblasts to varying titanium surface roughness. Biomaterials. 2011; 32(4):951–960.
Article26. Garcia AJ. Get a grip: integrins in cell-biomaterial interactions. Biomaterials. 2005; 26(36):7525–7529.
Article27. Huang Z, Nelson ER, Smith RL, Goodman SB. The sequential expression profiles of growth factors from osteoprogenitors [correction of osteroprogenitors] to osteoblasts in vitro. Tissue Eng. 2007; 13(9):2311–2320.
Article28. Dwivedi C, Gokhale S, Khim HG, Oh JK, Shon WY. Acetabular defect reconstruction with trabecular metal augments: study with minimum one-year follow-up. Hip Pelvis. 2017; 29(3):168–175.
Article29. Janecka IP. New reconstructive technologies in skull base surgery: role of titanium mesh and porous polyethylene. Arch Otolaryngol Head Neck Surg. 2000; 126(3):396–401.
Article30. Robinson Y, Tschoeke SK, Kayser R, Boehm H, Heyde CE. Reconstruction of large defects in vertebral osteomyelitis with expandable titanium cages. Int Orthop. 2009; 33(3):745–749.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoxia Pretreatment Promotes Chondrocyte Differentiation of Human Adipose-Derived Stem Cells via Vascular Endothelial Growth Factor
- Comparison of Cytocompatibility Between Grit Blasted Titanium Alloy (Ti-6Al-4V) with or without Pure Titanium Coating
- The Efficacy and Safety of Platelet-Rich Plasma and Adipose-Derived Stem Cells: An Update
- Osteogenic Differentiation of Human Adipose-derived Stem Cells within PLGA(Poly(D,L-lactic-co-glycolic acid)) Scaffold in the Nude Mouse
- Acceleration of Wound Healing Using Adipose-derived Stem Cell Therapy with Platelet Concentrates: Platelet-rich Plasma (PRP) vs. Platelet-rich Fibrin (PRF)