Ann Dermatol.
2019 Dec;31(6):611-620. 10.5021/ad.2019.31.6.611.
A Novel Effect of Lipids Extracted from Vernix Caseosa on Regulation of Filaggrin Expression in Human Epidermal Keratinocytes
- Affiliations
-
- 1Department of Marine Bio-Pharmacology, College of Food Science and Technology, Shanghai Ocean University, Shanghai, China.
- 2Pigeon Maternal & Infant Skin Care Research Institute, Shanghai, China. adolf@pigeon.cn
- KMID: 2461983
- DOI: http://doi.org/10.5021/ad.2019.31.6.611
Abstract
- BACKGROUND
Vernix caseosa (VC), which is known as a unique human substance, is a biofilm that covers the skin of most human newborns. VC has many biological functions including anti-infective, skin cleansing and skin barrier repair.
OBJECTIVE
In the study, we purpose to investigate the novel effect of lipids extracted from VC on the regulation of filaggrin (FLG) expression and anti-inflammation in normal human epidermal keratinocyte (NHEK) cells.
METHODS
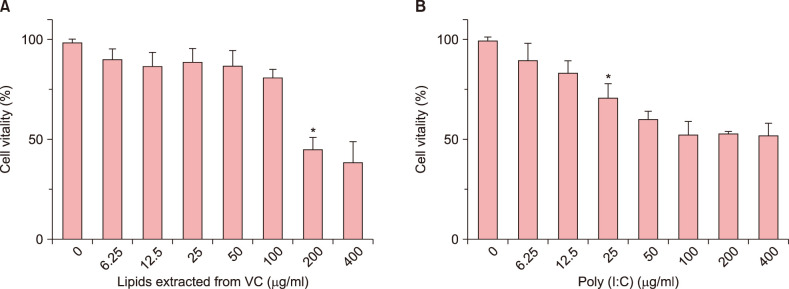
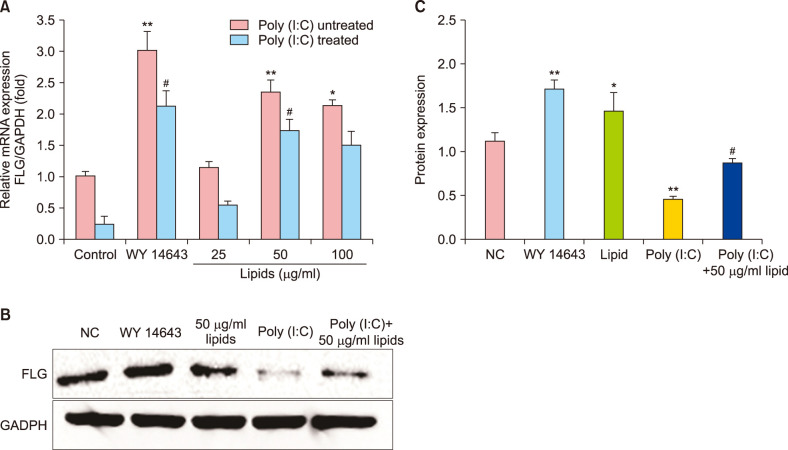
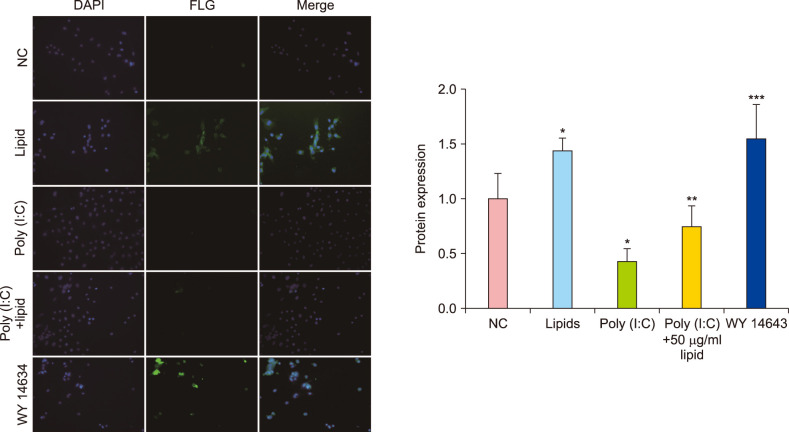
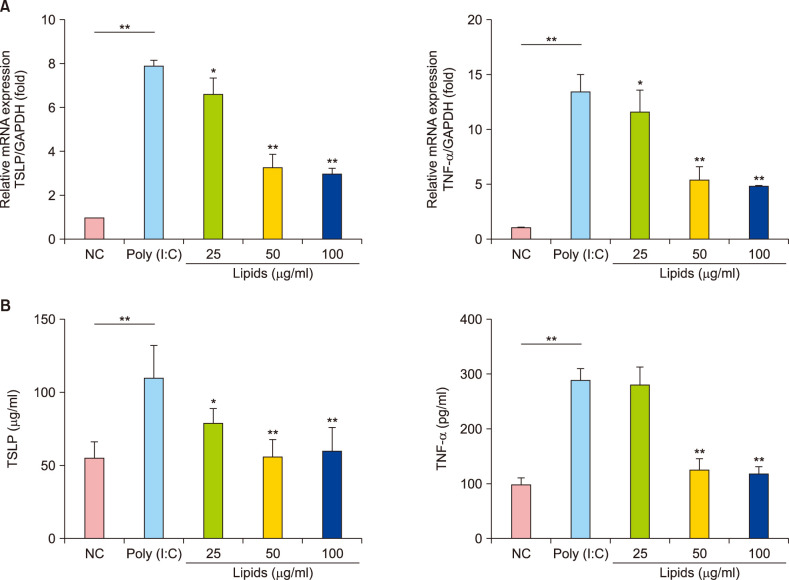
The lipids were extracted by chloroform/methanol (Folch method) and the major properties of fatty acid methyl esters were determined with gas chromatography-mass spectrometer. The relative viability of NHEK cells was evaluated by Cell Counting Kit 8 assay. The related expression of skin barrier protein was accessed with real-time quantitative polymerase chain reaction, Western blot and Immunofluorescence in NHEK cells with or without poly (I:C). Meanwhile, the changes of thymic stromal lymphopoietin (TSLP) and tumor necrosis factor alpha (TNF-α) are analyzed by enzyme-linked immunosorbent assay.
RESULTS
VC lipids mostly contained saturated and branched chains fatty acids. The expression of mRNA and protein of FLG were significantly increased after the supplement with lipid in NHEK cells. Meanwhile, lipids reversed the inhibition of poly (I:C) on FLG. Moreover, lipids suppressed the over secretion of TSLP and TNF-α induced by poly (I:C).
CONCLUSION
These results indicate that lipids extracted from VC has positive effects on the expression of FLG and anti-inflammation, suggesting that lipids of VC may be used for a reference for novel therapeutic method in reducing and remedying skin disease like atopic disease.
Keyword
MeSH Terms
-
Biofilms
Blotting, Western
Cell Count
Enzyme-Linked Immunosorbent Assay
Esters
Fatty Acids
Fluorescent Antibody Technique
Humans*
Infant, Newborn
Inflammation
Keratinocytes*
Methods
Polymerase Chain Reaction
RNA, Messenger
Skin
Skin Diseases
Tumor Necrosis Factor-alpha
Vernix Caseosa*
Esters
Fatty Acids
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Yamazaki Y, Nakamura Y, Núñez G. Role of the microbiota in skin immunity and atopic dermatitis. Allergol Int. 2017; 66:539–544. PMID: 28882556.
Article2. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006; 38:441–446. PMID: 16550169.3. Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012; 132(3 Pt 2):751–762. PMID: 22158554.
Article4. Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011; 365:1315–1327. PMID: 21991953.
Article5. Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011; 2:110. PMID: 21994899.
Article6. Brown JN, Kohler JJ, Coberley CR, Sleasman JW, Goodenow MM. HIV-1 activates macrophages independent of Toll-like receptors. PLoS One. 2008; 3:e3664. PMID: 19048100.
Article7. Sutmuller RP, Morgan ME, Netea MG, Grauer O, Adema GJ. Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol. 2006; 27:387–393. PMID: 16814607.
Article8. Hoath SB, Pickens WL, Visscher MO. The biology of vernix caseosa. Int J Cosmet Sci. 2006; 28:319–333. PMID: 18489296.
Article9. Tansirikongkol A, Wickett RR, Visscher MO, Hoath SB. Effect of vernix caseosa on the penetration of chymotryptic enzyme: potential role in epidermal barrier development. Pediatr Res. 2007; 62:49–53. PMID: 17515835.
Article10. Bautista MI, Wickett RR, Visscher MO, Pickens WL, Hoath SB. Characterization of vernix caseosa as a natural biofilm: comparison to standard oil-based ointments. Pediatr Dermatol. 2000; 17:253–260. PMID: 10990571.11. Visscher MO, Barai N, LaRuffa AA, Pickens WL, Narendran V, Hoath SB. Epidermal barrier treatments based on vernix caseosa. Skin Pharmacol Physiol. 2011; 24:322–329. PMID: 21822033.
Article12. Yan Y, Wang Z, Wang D, Lawrence P, Wang X, Kothapalli KSD, et al. BCFA-enriched vernix-monoacylglycerol reduces LPS-induced inflammatory markers in human enterocytes in vitro. Pediatr Res. 2018; 83:874–879. PMID: 29166379.
Article13. Fu HC, Nicolaides N. The structure of alkane diols of diesters in vernix caseosa lipids. Lipids. 1969; 4:170–175. PMID: 5782081.
Article14. Fluhr JW, Kao J, Jain M, Ahn SK, Feingold KR, Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001; 117:44–51. PMID: 11442748.
Article15. Bergsson G, Arnfinnsson J, Steingrímsson O, Thormar H. Killing of Gram-positive cocci by fatty acids and monoglycerides. APMIS. 2001; 109:670–678. PMID: 11890570.16. Boiten WA, Berkers T, Absalah S, van Smeden J, Lavrijsen APM, Bouwstra JA. Applying a vernix caseosa based formulation accelerates skin barrier repair by modulating lipid biosynthesis. J Lipid Res. 2018; 59:250–260. PMID: 29217624.
Article17. Tollin M, Bergsson G, Kai-Larsen Y, Lengqvist J, Sjövall J, Griffiths W, et al. Vernix caseosa as a multi-component defence system based on polypeptides, lipids and their interactions. Cell Mol Life Sci. 2005; 62:2390–2399. PMID: 16179970.
Article18. Bergsson G, Arnfinnsson J, Karlsson SM, Steingrímsson O, Thormar H. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1998; 42:2290–2294. PMID: 9736551.19. Wille JJ, Kydonieus A. Palmitoleic acid isomer (C16:1delta6) in human skin sebum is effective against gram-positive bacteria. Skin Pharmacol Appl Skin Physiol. 2003; 16:176–187. PMID: 12677098.20. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509. PMID: 13428781.
Article21. Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci. 2009; 122(Pt 9):1285–1294. PMID: 19386895.
Article22. Choi SY, Kim MJ, Ahn GR, Park KY, Lee MK, Seo SJ. The effect of adiponectin on the regulation of filaggrin expression in normal human epidermal keratinocytes. Ann Dermatol. 2018; 30:645–652.
Article23. Seguchi T, Cui CY, Kusuda S, Takahashi M, Aisu K, Tezuka T. Decreased expression of filaggrin in atopic skin. Arch Dermatol Res. 1996; 288:442–426. PMID: 8844122.
Article24. Tansirikongkol A, Visscher MO, Wickett RR. Water-handling properties of vernix caseosa and a synthetic analogue. J Cosmet Sci. 2007; 58:651–662. PMID: 18305878.25. Lee MS, Yoo YK, Lee KK. Preparation and characterization of vernix caseosa analogues. Korean J Aesthet Cosmetol. 2014; 12:547–553.26. Agrawal R, Woodfolk JA. Skin barrier defects in atopic dermatitis. Curr Allergy Asthma Rep. 2014; 14:433. PMID: 24633617.
Article27. Berkers T, van Dijk L, Absalah S, van Smeden J, Bouwstra JA. Topically applied fatty acids are elongated before incorporation in the stratum corneum lipid matrix in compromised skin. Exp Dermatol. 2017; 26:36–43. PMID: 27305861.
Article28. Wallmeyer L, Lehnen D, Eger N, Sochorová M, Opálka L, Kováčik A, et al. Stimulation of PPARα normalizes the skin lipid ratio and improves the skin barrier of normal and filaggrin deficient reconstructed skin. J Dermatol Sci. 2015; 80:102–110. PMID: 26472199.
Article29. Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006; 203:269–273. PMID: 16432252.
Article30. Míková R, Vrkoslav V, Hanus R, Háková E, Hábová Z, Doležal A, et al. Newborn boys and girls differ in the lipid composition of vernix caseosa. PLoS One. 2014; 9:e99173. PMID: 24911066.
Article31. Kim JH, Bae HC, Ko NY, Lee SH, Jeong SH, Lee H, et al. Thymic stromal lymphopoietin downregulates filaggrin expression by signal transducer and activator of transcription 3 (STAT3) and extracellular signal-regulated kinase (ERK) phosphorylation in keratinocytes. J Allergy Clin Immunol. 2015; 136:205–208.e209. PMID: 26026341.32. Miller LS, Modlin RL. Human keratinocyte Toll-like receptors promote distinct immune responses. J Invest Dermatol. 2007; 127:262–263. PMID: 17228303.
Article33. Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006; 24:341–348. PMID: 16546102.
Article34. Rouaud-Tinguely P, Boudier D, Marchand L, Barruche V, Bordes S, Coppin H, et al. From the morphological to the transcriptomic characterization of a compromised threedimensional in vitro model mimicking atopic dermatitis. Br J Dermatol. 2015; 173:1006–1014. PMID: 26147950.35. Dumortier A, Durham AD, Di Piazza M, Vauclair S, Koch U, Ferrand G, et al. Atopic dermatitis-like disease and associated lethal myeloproliferative disorder arise from loss of Notch signaling in the murine skin. PLoS One. 2010; 5:e9258. PMID: 20174635.
Article36. Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009; 15:1377–1382. PMID: 19966777.
Article37. Danso MO, van Drongelen V, Mulder A, van Esch J, Scott H, van Smeden J, et al. TNF-α and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J Invest Dermatol. 2014; 134:1941–1950. PMID: 24518171.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adiponectin Upregulates Filaggrin Expression via SIRT1-Mediated Signaling in Human Normal Keratinocytes
- Immunohistochemical Study of Expression of Involucrin, Loricrin, Filaggrin and Bcl-2 in Psoriasis
- Epigenetic Regulation of Filaggrin Gene Expression in Human Epidermal Keratinocytes
- Neonatal skin diseases
- The Effect of Adiponectin on the Regulation of Filaggrin Expression in Normal Human Epidermal Keratinocytes