Lab Anim Res.
2018 Dec;34(4):279-287. 10.5625/lar.2018.34.4.279.
CRISPR/Cas9-mediated generation of a Plac8 knockout mouse model
- Affiliations
-
- 1Department of Biochemistry, College of Life Science and Biotechnology and Yonsei Laboratory Animal Research Center, Yonsei University, Seoul, Korea. hwl@yonsei.ac.kr, jhlee13@gmail.com, rohjaeil@gmail.com
- 2Graduate School of Translational Medicine, Seoul National University College of Medicine, Seoul, Korea. bckang@snu.ac.kr
- 3Department of Experimental Animal Research, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 4Designed Animal and Transplantation Research Institute, Institute of GreenBio Science Technology, Seoul National University, Pyeongchang-gun, Korea.
- KMID: 2459306
- DOI: http://doi.org/10.5625/lar.2018.34.4.279
Abstract
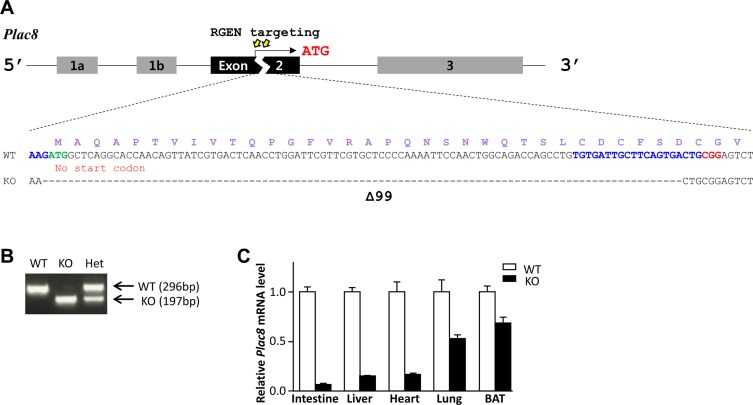
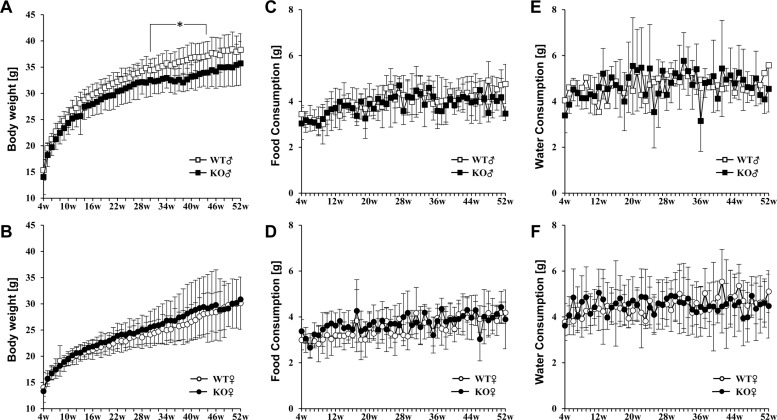
- Placenta specific 8 (PLAC8, also known as ONZIN) is a multi-functional protein that is highly expressed in the intestine, lung, spleen, and innate immune cells, and is involved in various diseases, including cancers, obesity, and innate immune deficiency. Here, we generated a Plac8 knockout mouse using the CRISPR/Cas9 system. The Cas9 mRNA and two single guide RNAs targeting a region near the translation start codon at Plac8 exon 2 were microinjected into mouse zygotes. This successfully eliminated the conventional translation start site, as confirmed by Sanger sequencing and PCR genotyping analysis. Unlike the previous Plac8 deficient models displaying increased adipose tissue and body weights, our male Plac8 knockout mice showed rather lower body weight than sex-matched littermate controls, though the only difference between these two mouse models is genetic context. Differently from the previously constructed embryonic stem cell-derived Plac8 knockout mouse that contains a neomycin resistance cassette, this knockout mouse model is free from a negative selection marker or other external insertions, which will be useful in future studies aimed at elucidating the multi-functional and physiological roles of PLAC8 in various diseases, without interference from exogenous foreign DNA.
Keyword
MeSH Terms
Figure
Reference
-
1. Artelt P, Grannemann R, Stocking C, Friel J, Bartsch J, Hauser H. The prokaryotic neomycin-resistance-encoding gene acts as a transcriptional silencer in eukaryotic cells. Gene. 1991; 99(2):249–254. PMID: 1850711.
Article2. Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, Chang DK, Cowley MJ, Gardiner BB, Song S, Harliwong I, Idrisoglu S, Nourse C, Nourbakhsh E, Manning S, Wani S, Gongora M, Pajic M, Scarlett CJ, Gill AJ, Pinho AV, Rooman I, Anderson M, Holmes O, Leonard C, Taylor D, Wood S, Xu Q, Nones K, Fink JL, Christ A, Bruxner T, Cloonan N, Kolle G, Newell F, Pinese M, Mead RS, Humphris JL, Kaplan W, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chou A, Chin VT, Chantrill LA, Mawson A, Samra JS, Kench JG, Lovell JA, Daly RJ, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N. Australian Pancreatic Cancer Genome Initiative. Kakkar N, Zhao F, Wu YQ, Wang M, Muzny DM, Fisher WE, Brunicardi FC, Hodges SE, Reid JG, Drummond J, Chang K, Han Y, Lewis LR, Dinh H, Buhay CJ, Beck T, Timms L, Sam M, Begley K, Brown A, Pai D, Panchal A, Buchner N, De Borja R, Denroche RE, Yung CK, Serra S, Onetto N, Mukhopadhyay D, Tsao MS, Shaw PA, Petersen GM, Gallinger S, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL, Morgan RA, Lawlor RT, Capelli P, Corbo V, Scardoni M, Tortora G, Tempero MA, Mann KM, Jenkins NA, Perez-Mancera PA, Adams DJ, Largaespada DA, Wessels LF, Rust AG, Stein LD, Tuveson DA, Copeland NG, Musgrove EA, Scarpa A, Eshleman JR, Hudson TJ, Sutherland RL, Wheeler DA, Pearson JV, McPherson JD, Gibbs RA, Grimmond SM. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012; 491(7424):399–405. PMID: 23103869.
Article3. Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, Kirchner T. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005; 179(1-2):56–65. PMID: 15942193.4. Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, Dickman MJ, Makarova KS, Koonin EV, van der Oost J. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008; 321(5891):960–964. PMID: 18703739.
Article5. Choi JY, Yun WB, Kim JE, Lee MR, Park JJ, Song BR, Kim HR, Park JW, Kang MJ, Kang BC, Lee HW, Hwang DY. Successful development of squamous cell carcinoma and hyperplasia in RGEN-mediated p27 KO mice after the treatment of DMBA and TPA. Lab Anim Res. 2018; 34(3):118–125. PMID: 30310408.
Article6. Choy MC, Visvanathan K, De Cruz P. An Overview of the Innate and Adaptive Immune System in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017; 23(1):2–13. PMID: 27779499.
Article7. Couillard C, Mauriège P, Imbeault P, Prud'homme D, Nadeau A, Tremblay A, Bouchard C, Després JP. Hyperleptinemia is more closely associated with adipose cell hypertrophy than with adipose tissue hyperplasia. Int J Obes Relat Metab Disord. 2000; 24(6):782–788. PMID: 10878687.
Article8. Doench JG, Fusi N, Sullender M, Hegde M, Vaimberg EW, Donovan KF, Smith I, Tothova Z, Wilen C, Orchard R, Virgin HW, Listgarten J, Root DE. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol. 2016; 34(2):184–191. PMID: 26780180.
Article9. Galaviz-Hernandez C, Stagg C, de Ridder G, Tanaka TS, Ko MS, Schlessinger D, Nagaraja R. Plac8 and Plac9, novel placental-enriched genes identified through microarray analysis. Gene. 2003; 309(2):81–89. PMID: 12758124.
Article10. Gukovsky I, Li N, Todoric J, Gukovskaya A, Karin M. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013; 144(6):1199–1209. PMID: 23622129.
Article11. Hickey MJ, Kubes P. Intravascular immunity: the host-pathogen encounter in blood vessels. Nat Rev Immunol. 2009; 9(5):364–375. PMID: 19390567.
Article12. Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013; 31(9):827–832. PMID: 23873081.
Article13. Jimenez-Preitner M, Berney X, Thorens B. Plac8 is required for white adipocyte differentiation in vitro and cell number control in vivo. PLoS One. 2012; 7(11):e48767. PMID: 23155406.
Article14. Jimenez-Preitner M, Berney X, Uldry M, Vitali A, Cinti S, Ledford JG, Thorens B. Plac8 is an inducer of C/EBPβ required for brown fat differentiation, thermoregulation, and control of body weight. Cell Metab. 2011; 14(5):658–670. PMID: 21982742.
Article15. Jimenez-Preitner M, Berney X, Uldry M, Vitali A, Cinti S, Ledford JG, Thorens B. Plac8 is an inducer of C/EBPβ required for brown fat differentiation, thermoregulation, and control of body weight. Cell Metab. 2011; 14(5):658–670. PMID: 21982742.
Article16. Kaistha BP, Lorenz H, Schmidt H, Sipos B, Pawlak M, Gierke B, Kreider R, Lankat-Buttgereit B, Sauer M, Fiedler L, Krattenmacher A, Geisel B, Kraus JM, Frese KK, Kelkenberg S, Giese NA, Kestler HA, Gress TM, Buchholz M. PLAC8 Localizes to the Inner Plasma Membrane of Pancreatic Cancer Cells and Regulates Cell Growth and Disease Progression through Critical Cell-Cycle Regulatory Pathways. Cancer Res. 2016; 76(1):96–107. PMID: 26669866.
Article17. Kang Y, Massagué J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004; 118(3):277–279. PMID: 15294153.18. Kim JH, Cho EY, Kwon E, Kim WH, Park JS, Lee YS, Yun JW, Kang BC. Gold thread implantation promotes hair growth in human and mice. Lab Anim Res. 2017; 33(4):291–297. PMID: 29399026.
Article19. Kinsey C, Balakrishnan V, O'Dell MR, Huang JL, Newman L, Whitney-Miller CL, Hezel AF, Land H. Plac8 links oncogenic mutations to regulation of autophagy and is critical to pancreatic cancer progression. Cell Rep. 2014; 7(4):1143–1155. PMID: 24794439.
Article20. Krzizek EC, Brix JM, Herz CT, Kopp HP, Schernthaner GH, Schernthaner G, Ludvik B. Prevalence of Micronutrient Deficiency in Patients with Morbid Obesity Before Bariatric Surgery. Obes Surg. 2018; 28(3):643–648. PMID: 28849358.
Article21. Lachmann PJ. Microbial subversion of the immune response. Proc Natl Acad Sci U S A. 2002; 99(13):8461–8462. PMID: 12077314.
Article22. Ledford JG, Kovarova M, Koller BH. Impaired host defense in mice lacking ONZIN. J Immunol. 2007; 178(8):5132–5143. PMID: 17404296.
Article23. Lee JH, Rho JI, Devkota S, Sung YH, Lee HW. Developing genetically engineered mouse models using engineered nucleases: Current status, challenges, and the way forward. Drug Discov Today Dis Models. 2016; 20:13–20.
Article24. Li C, Ma H, Wang Y, Cao Z, Graves-Deal R, Powell AE, Starchenko A, Ayers GD, Washington MK, Kamath V, Desai K, Gerdes MJ, Solnica-Krezel L, Coffey RJ. Excess PLAC8 promotes an unconventional ERK2-dependent EMT in colon cancer. J Clin Invest. 2014; 124(5):2172–2187. PMID: 24691442.
Article25. McMurray HR, Sampson ER, Compitello G, Kinsey C, Newman L, Smith B, Chen SR, Klebanov L, Salzman P, Yakovlev A, Land H. Synergistic response to oncogenic mutations defines gene class critical to cancer phenotype. Nature. 2008; 453(7198):1112–1116. PMID: 18500333.
Article26. Michl P, Gress TM. Current concepts and novel targets in advanced pancreatic cancer. Gut. 2013; 62(2):317–326. PMID: 23112132.
Article27. Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016; 66(4):271–289. PMID: 27253694.
Article28. Müller U. Ten years of gene targeting: targeted mouse mutants, from vector design to phenotype analysis. Mech Dev. 1999; 82(1-2):3–21. PMID: 10354467.
Article29. Paquet D, Kwart D, Chen A, Sproul A, Jacob S, Teo S, Olsen KM, Gregg A, Noggle S, Tessier-Lavigne M. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature. 2016; 533(7601):125–129. PMID: 27120160.
Article30. Roh JI, Cheong C, Sung YH, Lee J, Oh J, Lee BS, Lee JE, Gho YS, Kim DK, Park CB, Lee JH, Lee JW, Kang SM, Lee HW. Perturbation of NCOA6 leads to dilated cardiomyopathy. Cell Rep. 2014; 8(4):991–998. PMID: 25131203.
Article31. Roh JI, Kim Y, Oh J, Kim Y, Lee J, Lee J, Chun KH, Lee HW. Hexokinase 2 is a molecular bridge linking telomerase and autophagy. PLoS One. 2018; 13(2):e0193182. PMID: 29462198.
Article32. Roh JI, Lee J, Park SU, Kang YS, Lee J, Oh AR, Choi DJ, Cha JY, Lee HW. CRISPR-Cas9-mediated generation of obese and diabetic mouse models. Exp Anim. 2018; 67(2):229–237. PMID: 29343656.
Article33. Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005; 23:197–223. PMID: 15771570.
Article34. Sung YH, Kim JM, Kim HT, Lee J, Jeon J, Jin Y, Choi JH, Ban YH, Ha SJ, Kim CH, Lee HW, Kim JS. Highly efficient gene knockout in mice and zebrafish with RNA-guided endonucleases. Genome Res. 2014; 24(1):125–131. PMID: 24253447.
Article35. Valera A, Perales JC, Hatzoglou M, Bosch F. Expression of the neomycin-resistance (neo) gene induces alterations in gene expression and metabolism. Hum Gene Ther. 1994; 5(4):449–456. PMID: 7914094.
Article36. Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000; 80(5):617–653. PMID: 10830774.
Article37. Wright C, Simone NL. Obesity and tumor growth: inflammation, immunity, and the role of a ketogenic diet. Curr Opin Clin Nutr Metab Care. 2016; 19(4):294–299. PMID: 27168354.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Generation of knockout mouse models of cyclin-dependent kinase inhibitors by engineered nuclease-mediated genome editing
- CRISPR-Cas9 system in autosomal dominant polycystic kidney disease: a comprehensive review
- The length of guide RNA and target DNA heteroduplex effects on CRISPR/Cas9 mediated genome editing efficiency in porcine cells
- Genome editing: the road of CRISPR/Cas9 from bench to clinic
- Construction of a CRISPR/Cas9-Mediated Genome Editing System in Lentinula edodes