Lab Anim Res.
2018 Dec;34(4):232-238. 10.5625/lar.2018.34.4.232.
A comparison of metabolomic changes in type-1 diabetic C57BL/6N mice originating from different sources
- Affiliations
-
- 1College of Pharmacy, Pusan National University, Busan, Korea. youngjung@pusan.ac.kr
- 2College of Pharmacy, Brain Busan 21 Plus Program, Kyungsung University, Busan, Korea.
- 3Department of Pharmaceutical Engineering, Cheongju University, Cheongju, Korea.
- 4Department of Health and Exercise Science, Korea National Sport University, Seoul, Korea.
- 5College of Veterinary Medicine, Kyungpook National University, Daegu, Korea.
- 6College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea.
- KMID: 2459300
- DOI: http://doi.org/10.5625/lar.2018.34.4.232
Abstract
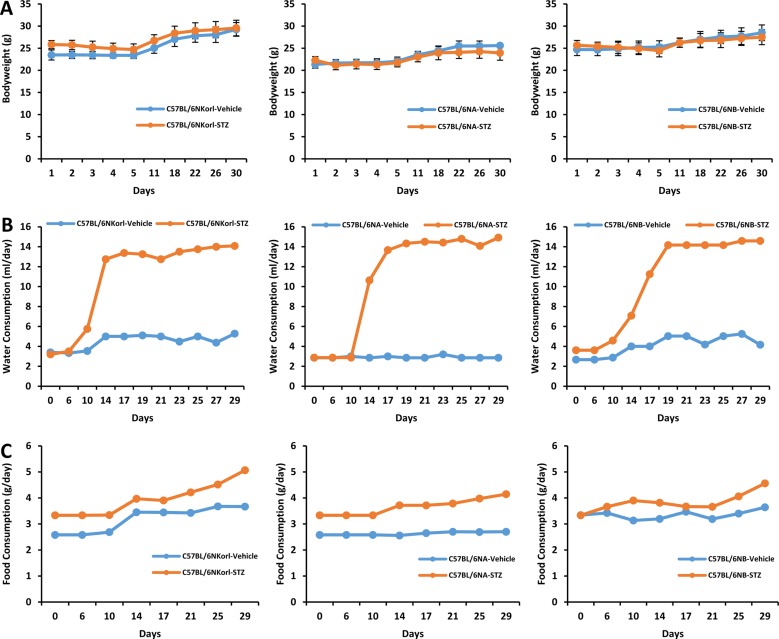
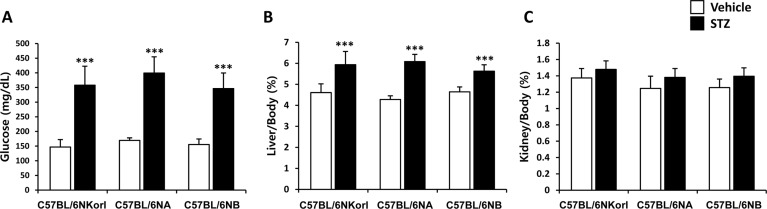
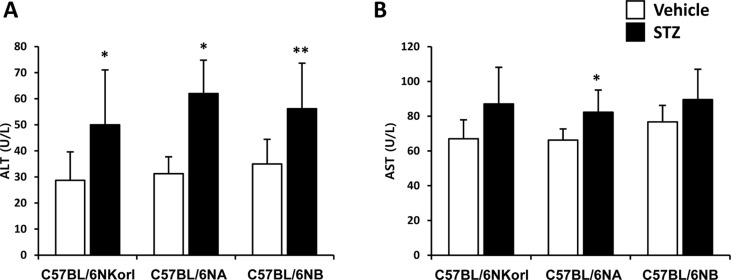
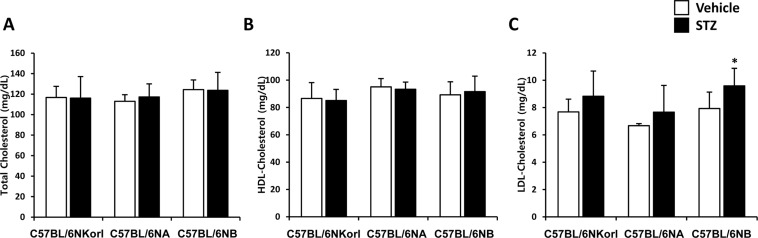
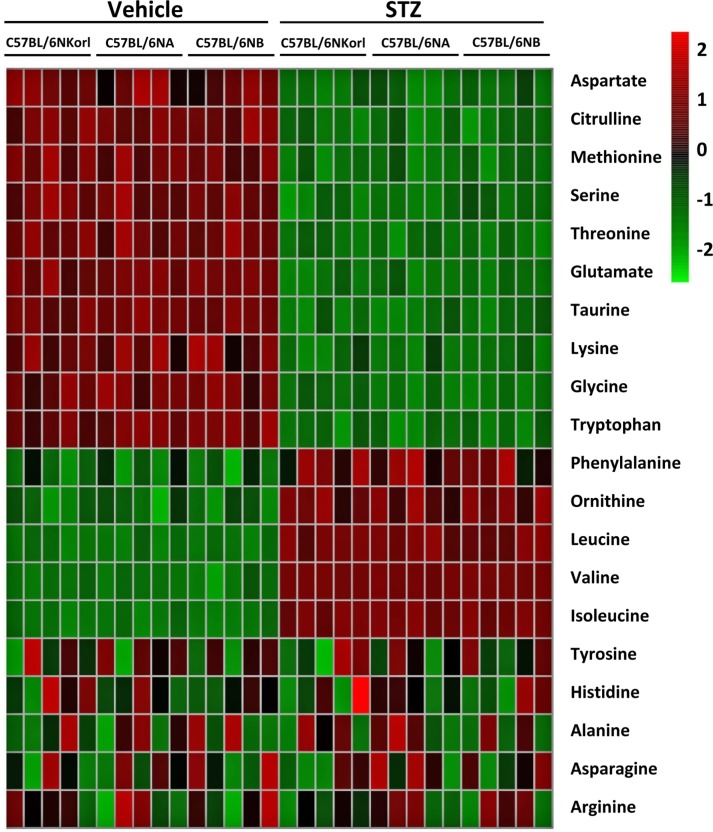
- Animal models have been used to elucidate the pathophysiology of varying diseases and to provide insight into potential targets for therapeutic intervention. Although alternatives to animal testing have been proposed to help overcome potential drawbacks related to animal experiments and avoid ethical issues, their use remains vital for the testing of new drug candidates and to identify the most effective strategies for therapeutic intervention. Particularly, the study of metabolic diseases requires the use of animal models to monitor whole-body physiology. In line with this, the National Institute of Food and Drug Safety Evaluation (NIFDS) in Korea has established their own animal strains to help evaluate both efficacy and safety during new drug development. The objective of this study was to characterize the response of C57BL/6NKorl mice from the NIFDS compared with that of other mice originating from the USA and Japan in a chemical-induced diabetic condition. Multiple low-dose treatments with streptozotocin were used to generate a type-1 diabetic animal model which is closely linked to the known clinical pathology of this disease. There were no significantly different responses observed between the varying streptozotocin-induced type-1 diabetic models tested in this study. When comparing control and diabetic mice, increases in liver weight and disturbances in serum amino acids levels of diabetic mice were most remarkable. Although the relationship between type-1 diabetes and BCAA has not been elucidated in this study, the results, which reveal a characteristic increase in diabetic mice of all origins are considered worthy of further study.
MeSH Terms
Figure
Reference
-
1. Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003; 17(1):24–38. PMID: 12616644.
Article2. American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2014; 37(Suppl 1):S81–S90. PMID: 24357215.3. Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton's Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008; 3(1):e1451. PMID: 18197261.
Article4. Diabetes Prevention Trial--Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002; 346(22):1685–1691. PMID: 12037147.5. van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011; 91(1):79–118. PMID: 21248163.
Article6. Knip M, Veijola R, Virtanen SM, Hyöty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005; 54(Suppl 2):S125–S136. PMID: 16306330.
Article7. Skyler JS, Cefalu WT, Kourides IA, Landschulz WH, Balagtas CC, Cheng SL, Gelfand RA. Efficacy of inhaled human insulin in type 1 diabetes mellitus: a randomised proof-of-concept study. Lancet. 2001; 357(9253):331–335. PMID: 11210993.
Article8. Polat K, Güneş S. An expert system approach based on principal component analysis and adaptive neuro-fuzzy inference system to diagnosis of diabetes disease. Digital Signal Processing. 2007; 17(4):702–710.
Article9. Oelze M, Knorr M, Schuhmacher S, Heeren T, Otto C, Schulz E, Reifenberg K, Wenzel P, Münzel T, Daiber A. Vascular dysfunction in streptozotocin-induced experimental diabetes strictly depends on insulin deficiency. J Vasc Res. 2011; 48(4):275–284. PMID: 21273782.
Article10. Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008; 51(2):216–226. PMID: 18087688.
Article11. Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001; 50(6):537–546. PMID: 11829314.12. Eleazu CO, Eleazu KC, Chukwuma S, Essien UN. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord. 2013; 12(1):60. PMID: 24364898.
Article13. Kolb H. Mouse models of insulin dependent diabetes: low-dose streptozocin-induced diabetes and nonobese diabetic (NOD) mice. Diabetes Metab Rev. 1987; 3(3):751–778. PMID: 2956075.
Article14. Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976; 193(4251):415–417. PMID: 180605.
Article15. Wu KK, Huan Y. Diabetic atherosclerosis mouse models. Atherosclerosis. 2007; 191(2):241–249. PMID: 16979174.
Article16. Weide LG, Lacy PE. Low-dose streptozocin-induced autoimmune diabetes in islet transplantation model. Diabetes. 1991; 40(9):1157–1162. PMID: 1834505.
Article17. REITMAN S, FRANKEL S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957; 28(1):56–63. PMID: 13458125.
Article18. Motyl K, McCabe LR. Streptozotocin, type I diabetes severity and bone. Biol Proced Online. 2009; 11:296–315. PMID: 19495918.
Article19. Fontaine DA, Davis DB. Attention to Background Strain Is Essential for Metabolic Research: C57BL/6 and the International Knockout Mouse Consortium. Diabetes. 2016; 65(1):25–33. PMID: 26696638.
Article20. Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004; 81(2):243–248. PMID: 15159170.
Article21. Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004; 53(Suppl 3):S215–S219. PMID: 15561913.22. Mashimo T, Serikawa T. Rat resources in biomedical research. Curr Pharm Biotechnol. 2009; 10(2):214–220. PMID: 19199954.
Article23. Croy BA. The 1999 Reginald Thomson Lecture. Custom-built mice: unique discovery tools in biomedical research. Can Vet J. 2000; 41(3):201–206. PMID: 10738597.24. Ardaillou R. [Transgenic mice: a major advance in biomedical research]. Bull Acad Natl Med. 2009; 193(8):1773–1782. PMID: 20669542.25. Sundberg JP, Schofield PN. Commentary: mouse genetic nomenclature. Standardization of strain, gene, and protein symbols. Vet Pathol. 2010; 47(6):1100–1104. PMID: 20685919.26. Junod A, Lambert AE, Stauffacher W, Renold AE. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest. 1969; 48(11):2129–2139. PMID: 4241908.
Article27. Junod A, Lambert AE, Orci L, Pictet R, Gonet AE, Renold AE. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med. 1967; 126(1):201–205. PMID: 4864021.
Article28. Furman BL. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr Protoc Pharmacol. 2015; 70:5.47.1–5.47.20.
Article29. Habibuddin M, Daghriri HA, Humaira T, Al Qahtani MS, Hefzi AA. Antidiabetic effect of alcoholic extract of Caralluma sinaica L. on streptozotocin-induced diabetic rabbits. J Ethnopharmacol. 2008; 117(2):215–220. PMID: 18359177.
Article30. Lee SI, Kim JS, Oh SH, Park KY, Lee HG, Kim SD. Antihyperglycemic effect of Fomitopsis pinicola extracts in streptozotocin-induced diabetic rats. J Med Food. 2008; 11(3):518–524. PMID: 18800901.
Article31. Merzouk H, Madani S, Chabane Sari D, Prost J, Bouchenak M, Belleville J. Time course of changes in serum glucose, insulin, lipids and tissue lipase activities in macrosomic offspring of rats with streptozotocin-induced diabetes. Clin Sci (Lond). 2000; 98(1):21–30. PMID: 10600655.
Article32. Ohno T, Horio F, Tanaka S, Terada M, Namikawa T, Kitoh J. Fatty liver and hyperlipidemia in IDDM (insulin-dependent diabetes mellitus) of streptozotocin-treated shrews. Life Sci. 2000; 66(2):125–131. PMID: 10666008.
Article33. Grill V, Björkman O, Gutniak M, Lindqvist M. Brain uptake and release of amino acids in nondiabetic and insulin-dependent diabetic subjects: important role of glutamine release for nitrogen balance. Metabolism. 1992; 41(1):28–32. PMID: 1538641.
Article34. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009; 9(4):311–326. PMID: 19356713.
Article35. Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB, Stevens RD, Muehlbauer MJ, Wenner BR, Bain JR, Laferrère B, Gorroochurn P, Teixeira J, Brantley PJ, Stevens VJ, Hollis JF, Appel LJ, Lien LF, Batch B, Newgard CB, Svetkey LP. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012; 55(2):321–330. PMID: 22065088.
Article36. McCormack SE, Shaham O, McCarthy MA, Deik AA, Wang TJ, Gerszten RE, Clish CB, Mootha VK, Grinspoon SK, Fleischman A. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2013; 8(1):52–61. PMID: 22961720.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Compensatory role of C3 convertase on the strain difference for C3 protein expression in FVB/N, C3H/HeN and C57BL/6N mice
- Comparison of humoral and cell-mediated immunity in three different C57BL/6N mouse substrains
- Use of C57BL/6N mice on the variety of immunological researches
- Comparative study of fertilization rates of C57BL/6NKorl and C57BL/6N mice obtained from two other sources
- Concurrent response to challenge infection with Cryptosporidium parvum in immunosuppressed C57BL/6N mice