Yonsei Med J.
2019 Sep;60(9):832-841. 10.3349/ymj.2019.60.9.832.
miR-1301/TRIAP1 Axis Participates in Epirubicin-Mediated Anti-Proliferation and Pro-Apoptosis in Osteosarcoma
- Affiliations
-
- 1Department of Pharmacy, Gansu Provincial Hospital, Lanzhou, Gansu, China.
- 2Department III of Orthopedic, Gansu Provincial Hospital, Lanzhou, Gansu, China. budalagong305@sina.com
- KMID: 2457479
- DOI: http://doi.org/10.3349/ymj.2019.60.9.832
Abstract
- PURPOSE
Epirubicin is one of the most effective drugs against osteosarcoma. miR-1301 is involved in the occurrence and development of osteosarcoma. Whether miR-1301 is responsible for the chemosensitivity of osteosarcoma cells to epirubicin remains largely unknown.
MATERIALS AND METHODS
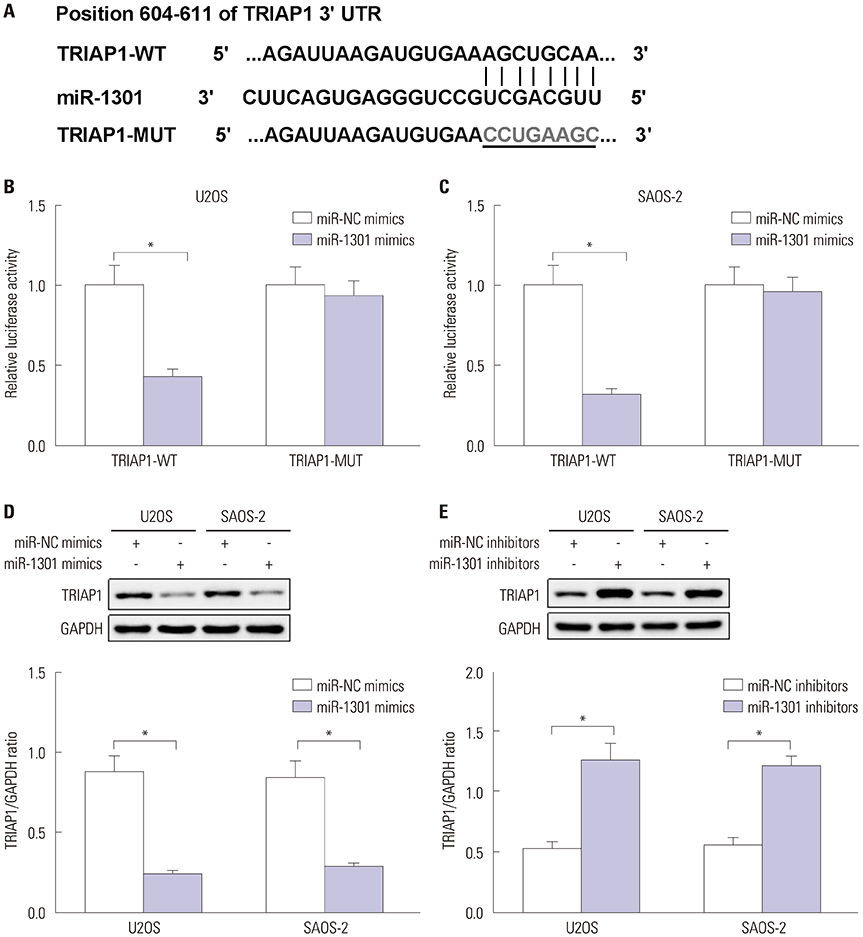
U2OS and SAOS-2 cells were treated with various concentrations of epirubicin. Flow cytometry was employed to evaluate cell apoptotic rate. Cell proliferation was measured by Cell Counting Kit-8 assay. Western blot and quantitative real-time polymerase chain reaction were utilized to detect the expressions of B-cell lymphoma-2 (Bcl-2), Bcl-2 assaciated X protein (Bax), cleaved-caspase-3, cleaved-poly (ADP-ribose) polymerases (PARP1), TP53-regulated inhibitor of apoptosis 1 (TRIAP1), and microRNA-1301 (miR-1301). The relationship between miR-1301 and TRIAP1 was determined by luciferase reporter assay.
RESULTS
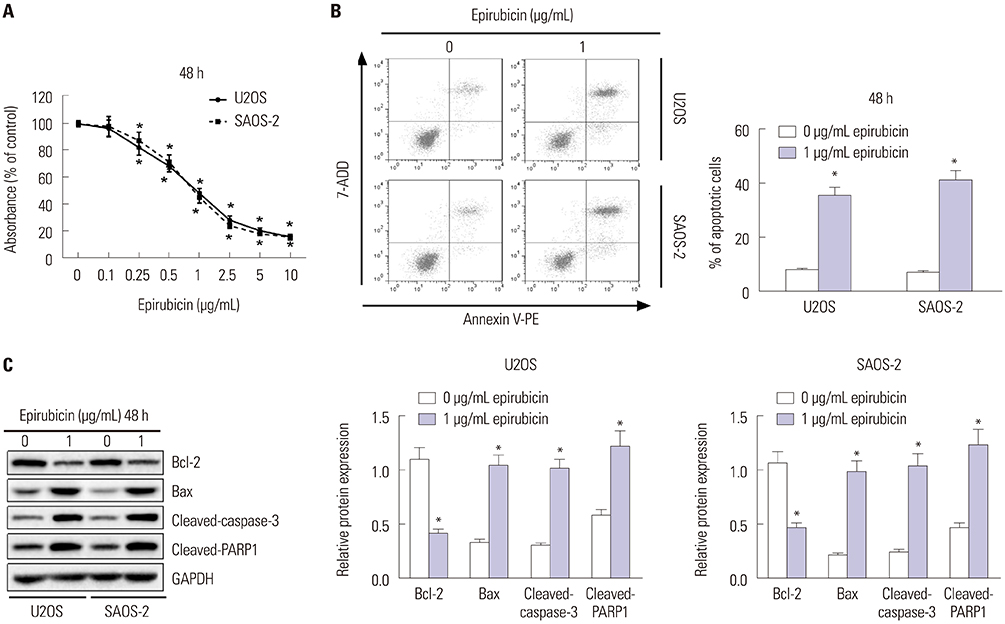
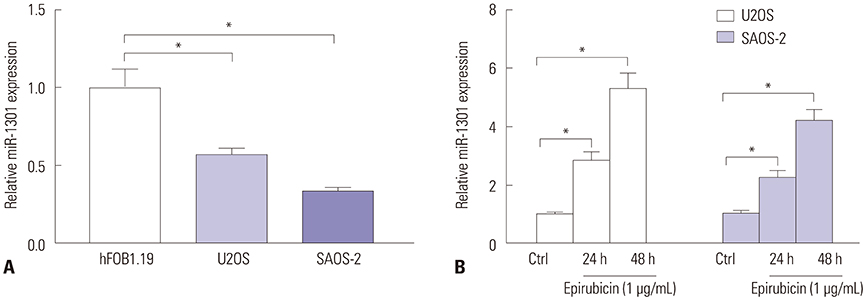
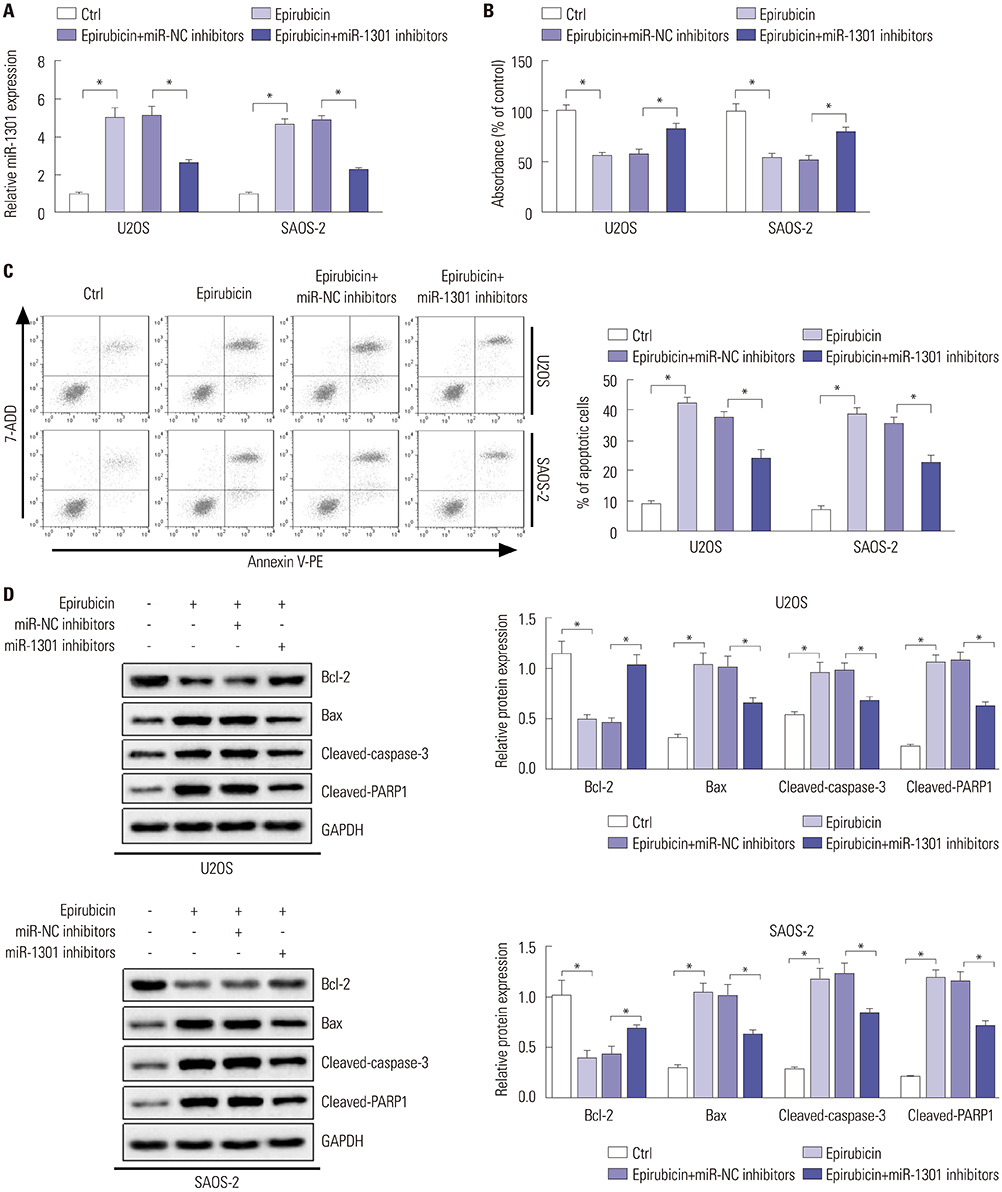
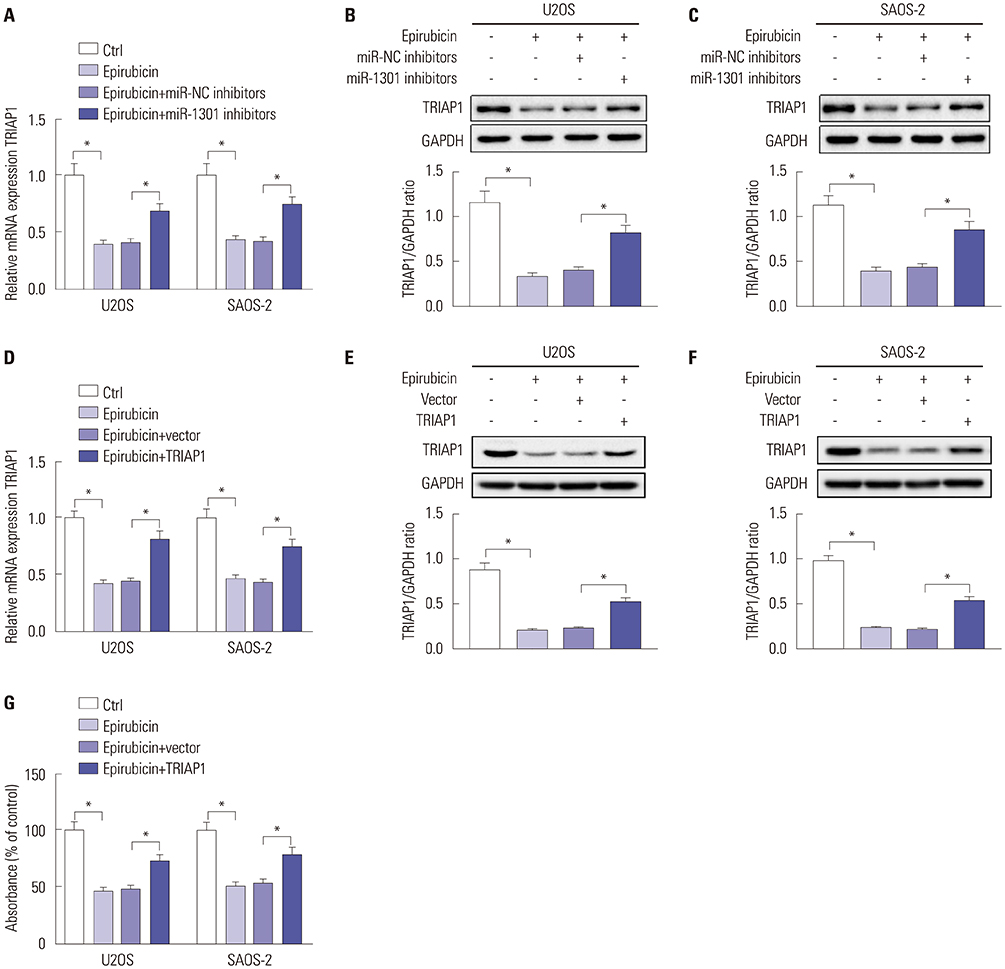
Epirubicin inhibited proliferation in a dose-dependent manner, induced apoptosis, decreased the expression of Bcl-2, and increased the expressions of Bax, cleaved-caspase-3, and cleaved-PARP1 in osteosarcoma cells. miR-1301 was downregulated in U2OS and SAOS-2 cells. Importantly, epirubicin significantly increased the levels of miR-1301. Overexpression of miR-1301 suppressed proliferation and promoted apoptosis. Interestingly, those effects were enhanced by epirubicin. In contrast, miR-1301 depletion attenuated the epirubicin-mediated anti-osteosarcoma effect. miR-1301 negatively regulated the expression of TRIAP1 in U2OS and SAOS-2 cells. Furthermore, epirubicin inhibited the mRNA and protein levels of TRIAP1 by upregulating miR-1301 levels. Epirubicin suppressed cell proliferation by downregulating TRIAP1.
CONCLUSION
miR-1301 was implicated in the chemosensitivity of osteosarcoma to epirubicin by modulating TRIAP1.
Keyword
MeSH Terms
Figure
Reference
-
1. Moore DD, Luu HH. Osteosarcoma. Cancer Treat Res. 2014; 162:65–92.
Article2. Biazzo A, De Paolis M. Multidisciplinary approach to osteosarcoma. Acta Orthop Belg. 2016; 82:690–698.3. Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018; 18:39–50.
Article4. Fan XL, Cai GP, Zhu LL, Ding GM. Efficacy and safety of ifosfamide-based chemotherapy for osteosarcoma: a meta-analysis. Drug Des Devel Ther. 2015; 9:5925–5932.
Article5. Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment - where do we stand? A state of the art review. Cancer Treat Rev. 2014; 40:523–532.
Article6. Friebele JC, Peck J, Pan X, Abdel-Rasoul M, Mayerson JL. Osteosarcoma: a meta-analysis and review of the literature. Am J Orthop (Belle Mead NJ). 2015; 44:547–553.7. Petrioli R, Roviello G, Zanotti L, Roviello F, Polom K, Bottini A, et al. Epirubicin-based compared with docetaxel-based chemotherapy for advanced gastric carcinoma: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016; 102:82–88.
Article8. Wu J, Xue X, Zhang B, Cao H, Kong F, Jiang W, et al. Enhanced antitumor activity and attenuated cardiotoxicity of Epirubicin combined with Paeonol against breast cancer. Tumour Biol. 2016; 37:12301–12313.
Article9. Liu ZL, Wang G, Shu Y, Zou PA, Zhou Y, Yin QS. Enhanced antitumor activity of epirubicin combined with cerulenin in osteosarcoma. Mol Med Rep. 2012; 5:326–330.10. Basaran M, Bavbek ES, Saglam S, Eralp L, Sakar B, Atalar AC, et al. A phase II study of cisplatin, ifosfamide and epirubicin combination chemotherapy in adults with nonmetastatic and extremity osteosarcomas. Oncology. 2007; 72:255–260.
Article11. Acunzo M, Romano G, Wernicke D, Croce CM. MicroRNA and cancer--a brief overview. Adv Biol Regul. 2015; 57:1–9.12. Waresijiang N, Sun J, Abuduaini R, Jiang T, Zhou W, Yuan H. The downregulation of miR-125a-5p functions as a tumor suppressor by directly targeting MMP-11 in osteosarcoma. Mol Med Rep. 2016; 13:4859–4864.
Article13. Bach DH, Hong JY, Park HJ, Lee SK. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int J Cancer. 2017; 141:220–230.
Article14. Wang J, Yang M, Li Y, Han B. The role of MicroRNAs in the chemoresistance of breast cancer. Drug Dev Res. 2015; 76:368–374.
Article15. Li Q, Liu M, Ma F, Luo Y, Cai R, Wang L, et al. Circulating miR-19a and miR-205 in serum may predict the sensitivity of luminal A subtype of breast cancer patients to neoadjuvant chemotherapy with epirubicin plus paclitaxel. PLoS One. 2014; 9:e104870.
Article16. Tan X, Fu Y, Chen L, Lee W, Lai Y, Rezaei K, et al. miR-671-5p inhibits epithelial-to-mesenchymal transition by downregulating FOXM1 expression in breast cancer. Oncotarget. 2016; 7:293–307.
Article17. Zhang W, Qiao B, Fan J. Overexpression of miR-4443 promotes the resistance of non-small cell lung cancer cells to epirubicin by targeting INPP4A and regulating the activation of JAK2/STAT3 pathway. Pharmazie. 2018; 73:386–392.18. Chen D, Liu D, Chen Z. Potential therapeutic implications of miRNAs in osteosarcoma chemotherapy. Tumour Biol. 2017; 39:1010428317705762.
Article19. Wang F, Yu D, Liu Z, Wang R, Xu Y, Cui H, et al. MiR-125b functions as a tumor suppressor and enhances chemosensitivity to cisplatin in osteosarcoma. Technol Cancer Res Treat. 2016; 15:NP105–NP112.
Article20. Zhang Y, Hu H, Song L, Cai L, Wei R, Jin W. Epirubicin-mediated expression of miR-302b is involved in osteosarcoma apoptosis and cell cycle regulation. Toxicol Lett. 2013; 222:1–9.
Article21. Wang L, Hu K, Chao Y. MicroRNA-1301 inhibits migration and invasion of osteosarcoma cells by targeting BCL9. Gene. 2018; 679:100–107.
Article22. Li Y, Tang X, He Q, Yang X, Ren X, Wen X, et al. Overexpression of mitochondria mediator gene TRIAP1 by miR-320b loss is associated with progression in nasopharyngeal carcinoma. PLoS Genet. 2016; 12:e1006183.
Article23. Zhi T, Jiang K, Zhang C, Xu X, Wu W, Nie E, et al. MicroRNA-1301 inhibits proliferation of human glioma cells by directly targeting N-Ras. Am J Cancer Res. 2017; 7:982–998.24. Peng X, Yan B, Shen Y. MiR-1301-3p inhibits human breast cancer cell proliferation by regulating cell cycle progression and apoptosis through directly targeting ICT1. Breast Cancer. 2018; 25:742–752.
Article25. Kvansakul M, Caria S, Hinds MG. The Bcl-2 family in host-virus interactions. Viruses. 2017; 9:E290.
Article26. Raemy E, Martinou JC. Involvement of cardiolipin in tBID-induced activation of BAX during apoptosis. Chem Phys Lipids. 2014; 179:70–74.
Article27. Choudhary GS, Al-Harbi S, Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol. 2015; 1219:1–9.
Article28. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007; 35:495–516.
Article29. Dong X, Yang L, Wang H. miR-520 promotes DNA-damage-induced trophoblast cell apoptosis by targeting PARP1 in recurrent spontaneous abortion (RSA). Gynecol Endocrinol. 2017; 33:274–278.
Article30. Adams C, Cazzanelli G, Rasul S, Hitchinson B, Hu Y, Coombes RC, et al. Apoptosis inhibitor TRIAP1 is a novel effector of drug resistance. Oncol Rep. 2015; 34:415–422.
Article31. Fook-Alves VL, de Oliveira MB, Zanatta DB, Strauss BE, Colleoni GW. TP53 Regulated Inhibitor of Apoptosis 1 (TRIAP1) stable silencing increases late apoptosis by upregulation of caspase 9 and APAF1 in RPMI8226 multiple myeloma cell line. Biochim Biophys Acta. 2016; 1862:1105–1110.
Article32. Liu P, Qi X, Bian C, Yang F, Lin X, Zhou S, et al. MicroRNA-18a inhibits ovarian cancer growth via directly targeting TRIAP1 and IPMK. Oncol Lett. 2017; 13:4039–4046.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Knockdown of Long Non-Coding RNA NEAT1 Inhibits Proliferation and Invasion and Induces Apoptosis of Osteosarcoma by Inhibiting miR-194 Expression
- Curcumin Inhibits the Proliferation, Migration, Invasion, and Apoptosis of Diffuse Large B-Cell Lymphoma Cell Line by Regulating MiR-21/VHL Axis
- Long Non-Coding RNA TUG1 Promotes Proliferation and Inhibits Apoptosis of Osteosarcoma Cells by Sponging miR-132-3p and Upregulating SOX4 Expression
- Autophagy Inhibition Promotes Quercetin Induced Apoptosis in MG-63 Human Osteosarcoma cells
- LncRNA LINC00313 Knockdown InhibitsTumorigenesis and Metastasis in HumanOsteosarcoma by Upregulating FOSL2through Sponging miR-342-3p