Yonsei Med J.
2018 Mar;59(2):226-235. 10.3349/ymj.2018.59.2.226.
Long Non-Coding RNA TUG1 Promotes Proliferation and Inhibits Apoptosis of Osteosarcoma Cells by Sponging miR-132-3p and Upregulating SOX4 Expression
- Affiliations
-
- 1Department of Orthopedics, The First Affiliated Hospital of the Medical College, Shihezi University, Shihezi, China. liganghavst@163.com
- KMID: 2418785
- DOI: http://doi.org/10.3349/ymj.2018.59.2.226
Abstract
- PURPOSE
Long non-coding RNA taurine upregulated gene 1 (TUG1) is reported to be a vital regulator of the progression of various cancers. This study aimed to explore the exact roles and molecular mechanisms of TUG1 in osteosarcoma (OS) development.
MATERIALS AND METHODS
Real-time quantitative PCR was applied to detect the expressions of TUG1 and microRNA-132-3p (miR-132-3p) in OS tissues and cells. Western blot was performed to measure protein levels of sex determining region Y-box 4 (SOX4). Cell viability was assessed using XTT assay. Cell apoptosis was evaluated using flow cytometry and caspase-3 activity detection assays. Bioinformatics analysis and luciferase reporter experiments were employed to confirm relationships among TUG1, miR-132-3p, and SOX4.
RESULTS
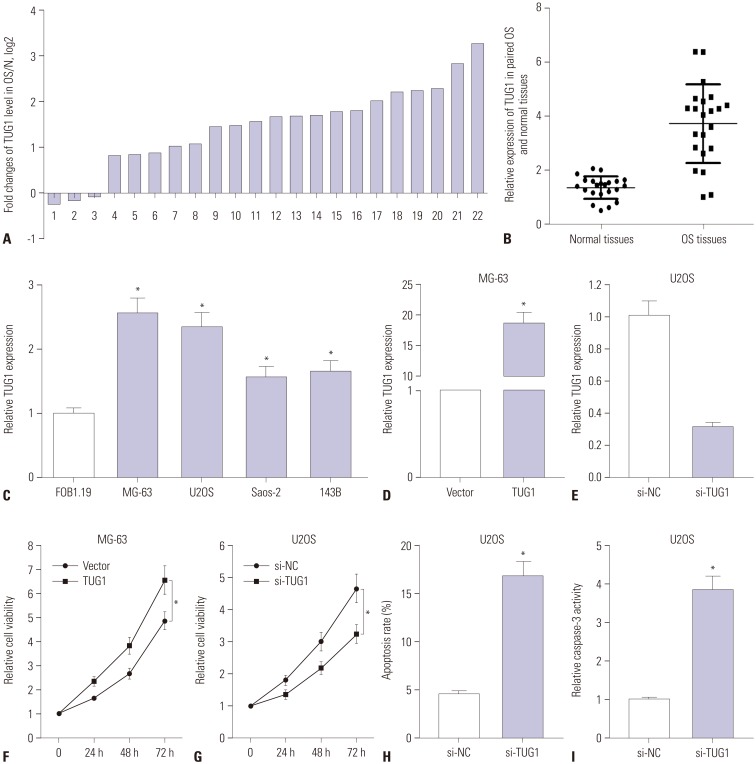
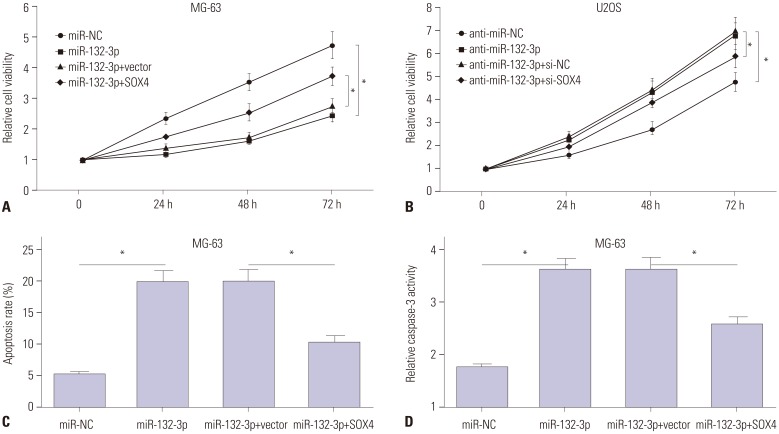
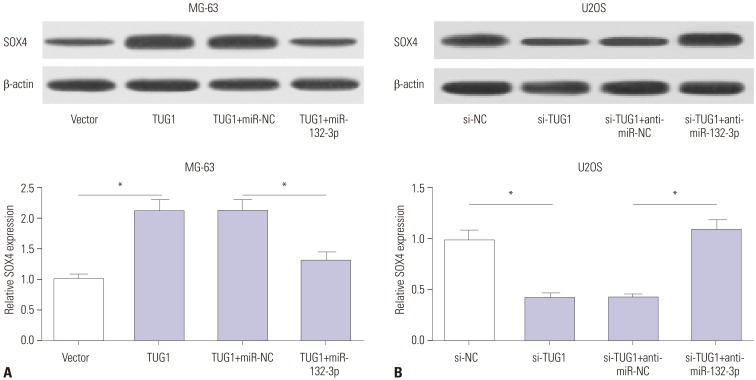
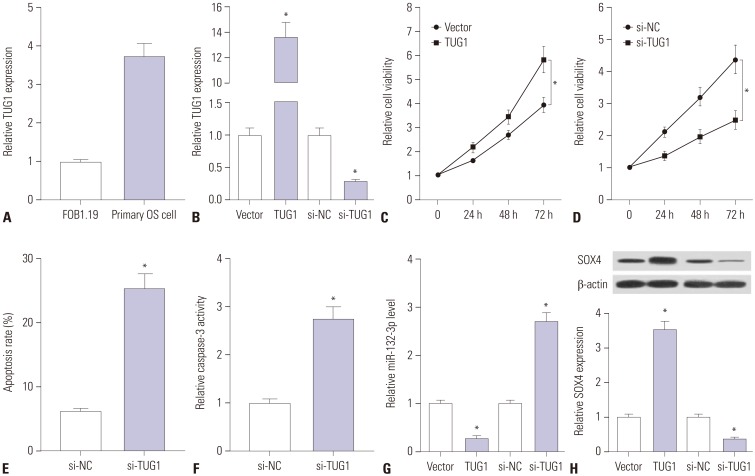
TUG1 was highly expressed in human OS tissues, OS cell lines, and primary OS cells. TUG1 knockdown hindered proliferation and induced apoptosis in human OS cell lines and primary OS cells. Moreover, TUG1 inhibited miR-132-3p expression by direct interaction, and introduction of miR-132-3p inhibitor partly abrogated the effect of TUG1 knockdown on the proliferation and apoptosis of OS cells. Furthermore, SOX4 was validated as a target of miR-132-3p. Further functional analyses revealed that miR-132-3p inhibited proliferation and induced apoptosis of OS cells, while this effect was greatly abated following SOX4 overexpression. Moreover, TUG1 knockdown suppressed proliferation and promoted apoptosis by upregulating miR-132-3p and downregulating SOX4 in primary OS cells.
CONCLUSION
TUG1 facilitated proliferation and suppressed apoptosis by regulating the miR-132-3p/SOX4 axis in human OS cell lines and primary OS cells. This finding provides a potential target for OS therapy.
Keyword
MeSH Terms
-
Apoptosis/*genetics
Biomarkers, Tumor
Bone Neoplasms/genetics/metabolism/*pathology
Cell Line, Tumor
Cell Proliferation
Gene Expression Regulation, Neoplastic/genetics
Gene Knockdown Techniques
Humans
MicroRNAs/*genetics/metabolism
Osteosarcoma/genetics/metabolism/*pathology
RNA, Long Noncoding/*genetics/metabolism
Real-Time Polymerase Chain Reaction
Reverse Transcriptase Polymerase Chain Reaction
SOXC Transcription Factors/genetics/*metabolism
Transcriptional Activation
Tumor Cells, Cultured
Up-Regulation
Biomarkers, Tumor
MicroRNAs
RNA, Long Noncoding
SOXC Transcription Factors
Figure
Reference
-
1. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009; 115:1531–1543. PMID: 19197972.2. Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children's Oncology Group. J Clin Oncol. 2008; 26:633–638. PMID: 18235123.
Article3. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011; 12:861–874. PMID: 22094949.
Article4. Catto JW, Alcaraz A, Bjartell AS, De Vere White R, Evans CP, Fussel S, et al. MicroRNA in prostate, bladder, and kidney cancer: a systematic review. Eur Urol. 2011; 59:671–681. PMID: 21296484.
Article5. Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014; 1839:1097–1109. PMID: 25159663.
Article6. Zhou G, Shi X, Zhang J, Wu S, Zhao J. MicroRNAs in osteosarcoma: from biological players to clinical contributors, a review. J Int Med Res. 2013; 41:1–12. PMID: 23569124.
Article7. Li Z, Yu X, Shen J. Long non-coding RNAs: emerging players in osteosarcoma. Tumour Biol. 2016; 37:2811–2816. PMID: 26718212.
Article8. Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014; 505:344–352. PMID: 24429633.
Article9. Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011; 146:353–358. PMID: 21802130.
Article10. Han Y, Liu Y, Gui Y, Cai Z. Long intergenic non-coding RNA TUG1 is overexpressed in urothelial carcinoma of the bladder. J Surg Oncol. 2013; 107:555–559. PMID: 22961206.
Article11. Xu Y, Wang J, Qiu M, Xu L, Li M, Jiang F, et al. Upregulation of the long noncoding RNA TUG1 promotes proliferation and migration of esophageal squamous cell carcinoma. Tumour Biol. 2015; 36:1643–1651. PMID: 25366138.
Article12. Zhang EB, Yin DD, Sun M, Kong R, Liu XH, You LH, et al. P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis. 2014; 5:e1243. PMID: 24853421.
Article13. Ma B, Li M, Zhang L, Huang M, Lei JB, Fu GH, et al. Upregulation of long non-coding RNA TUG1 correlates with poor prognosis and disease status in osteosarcoma. Tumour Biol. 2016; 37:4445–4455. PMID: 26499949.
Article14. Zhang Q, Geng PL, Yin P, Wang XL, Jia JP, Yao J. Down-regulation of long non-coding RNA TUG1 inhibits osteosarcoma cell proliferation and promotes apoptosis. Asian Pac J Cancer Prev. 2013; 14:2311–2315. PMID: 23725133.
Article15. Yun-Bo F, Xiao-Po L, Xiao-Li L, Guo-Long C, Pei Z, Fa-Ming T. LncRNA TUG1 is upregulated and promotes cell proliferation in osteosarcoma. Open Med (Wars). 2016; 11:163–167. PMID: 28352787.
Article16. Liu Y, Li Y, Liu J, Wu Y, Zhu Q. MicroRNA-132 inhibits cell growth and metastasis in osteosarcoma cell lines possibly by targeting Sox4. Int J Oncol. 2015; 47:1672–1684. PMID: 26352673.
Article17. Wang J, Xu G, Shen F, Kang Y. miR-132 targeting cyclin E1 suppresses cell proliferation in osteosarcoma cells. Tumour Biol. 2014; 35:4859–4865. PMID: 24449507.
Article18. Luo XJ, Tang DG, Gao TL, Zhang YL, Wang M, Quan ZX, et al. MicroRNA-212 inhibits osteosarcoma cells proliferation and invasion by down-regulation of Sox4. Cell Physiol Biochem. 2014; 34:2180–2188. PMID: 25562164.
Article19. Wu X, Zhou H, Yue B, Li M, Liu F, Qiu C, et al. Upregulation of microRNA-25-3p inhibits proliferation, migration and invasion of osteosarcoma cells in vitro by directly targeting SOX4. Mol Med Rep. 2017; 16:4293–4300. PMID: 28765961.
Article20. Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010; 21(Suppl 7):vii320–vii325. PMID: 20943636.
Article21. Yang Z, Li X, Yang Y, He Z, Qu X, Zhang Y. Long noncoding RNAs in the progression, metastasis, and prognosis of osteosarcoma. Cell Death Dis. 2016; 7:e2389. PMID: 27685633.
Article22. Dong Y, Liang G, Yuan B, Yang C, Gao R, Zhou X. MALAT1 promotes the proliferation and metastasis of osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol. 2015; 36:1477–1486. PMID: 25431257.
Article23. Wang Y, Yao J, Meng H, Yu Z, Wang Z, Yuan X, et al. A novel long non-coding RNA, hypoxia-inducible factor-2α promoter upstream transcript, functions as an inhibitor of osteosarcoma stem cells in vitro. Mol Med Rep. 2015; 11:2534–2540. PMID: 25434862.
Article24. Yang J, Gao T, Tang J, Cai H, Lin L, Fu S. Loss of microRNA-132 predicts poor prognosis in patients with primary osteosarcoma. Mol Cell Biochem. 2013; 381:9–15. PMID: 23801049.
Article25. Vlachos IS, Paraskevopoulou MD, Karagkouni D, Georgakilas G, Vergoulis T, Kanellos I, et al. DIANA-TarBase v7.0: indexing more than half a million experimentally supported miRNA:mRNA interactions. Nucleic Acids Res. 2015; 43:D153–D159. PMID: 25416803.
Article26. Liao YL, Sun YM, Chau GY, Chau YP, Lai TC, Wang JL, et al. Identification of SOX4 target genes using phylogenetic footprinting-based prediction from expression microarrays suggests that overexpression of SOX4 potentiates metastasis in hepatocellular carcinoma. Oncogene. 2008; 27:5578–5589. PMID: 18504433.
Article27. Liu P, Ramachandran S, Ali Seyed M, Scharer CD, Laycock N, Dalton WB, et al. Sex-determining region Y box 4 is a transforming oncogene in human prostate cancer cells. Cancer Res. 2006; 66:4011–4019. PMID: 16618720.
Article28. Xie CH, Cao YM, Huang Y, Shi QW, Guo JH, Fan ZW, et al. Long non-coding RNA TUG1 contributes to tumorigenesis of human osteosarcoma by sponging miR-9-5p and regulating POU2F1 expression. Tumour Biol. 2016; 37:15031–15041. PMID: 27658774.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Knockdown of Long Non-Coding RNA NEAT1 Inhibits Proliferation and Invasion and Induces Apoptosis of Osteosarcoma by Inhibiting miR-194 Expression
- LncRNA LINC00313 Knockdown InhibitsTumorigenesis and Metastasis in HumanOsteosarcoma by Upregulating FOSL2through Sponging miR-342-3p
- LncRNA Taurine-Upregulated Gene 1 Promotes Cell Proliferation by Inhibiting MicroRNA-9 in MCF-7 Cells
- LncRNA XLOC_006390 facilitates cervical cancer tumorigenesis and metastasis as a ceRNA against miR-331-3p and miR-338-3p
- Long Non-Coding RNA TUG1 Attenuates Insulin Resistance in Mice with Gestational Diabetes Mellitus via Regulation of the MicroRNA-328-3p/SREBP-2/ERK Axis