Korean J Radiol.
2016 Aug;17(4):497-508. 10.3348/kjr.2016.17.4.497.
Ultrasound-Guided Delivery of siRNA and a Chemotherapeutic Drug by Using Microbubble Complexes: In Vitro and In Vivo Evaluations in a Prostate Cancer Model
- Affiliations
-
- 1Department of Radiology, Seoul National University Bundang Hospital, Seongnam 13620, Korea. hakjlee@snu.ac.kr
- 2Department of Radiology, Seoul National University College of Medicine, Seoul 03080, Korea.
- 3Program in Nano Science and Technology, Department of Transdisciplinary Studies, Seoul National University Graduate School of Convergence Science and Technology, Suwon 16229, Korea.
- 4Department of Applied Bioscience, College of Life Science, CHA University, Pocheon 11160, Korea.
- 5College of Pharmacy, Ajou University, Suwon 16499, Korea.
- KMID: 2455420
- DOI: http://doi.org/10.3348/kjr.2016.17.4.497
Abstract
OBJECTIVE
To evaluate the effectiveness of ultrasound and microbubble-liposome complex (MLC)-mediated delivery of siRNA and doxorubicin into prostate cancer cells and its therapeutic capabilities both in vitro and in vivo.
MATERIALS AND METHODS
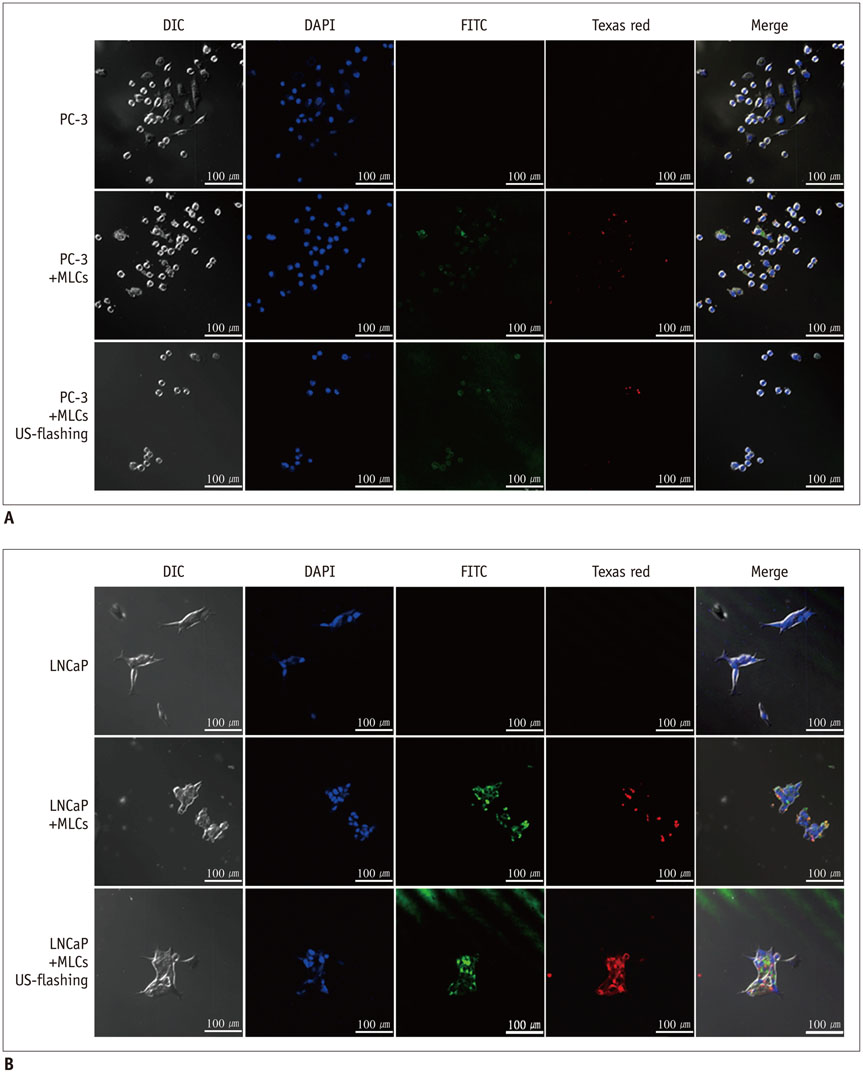
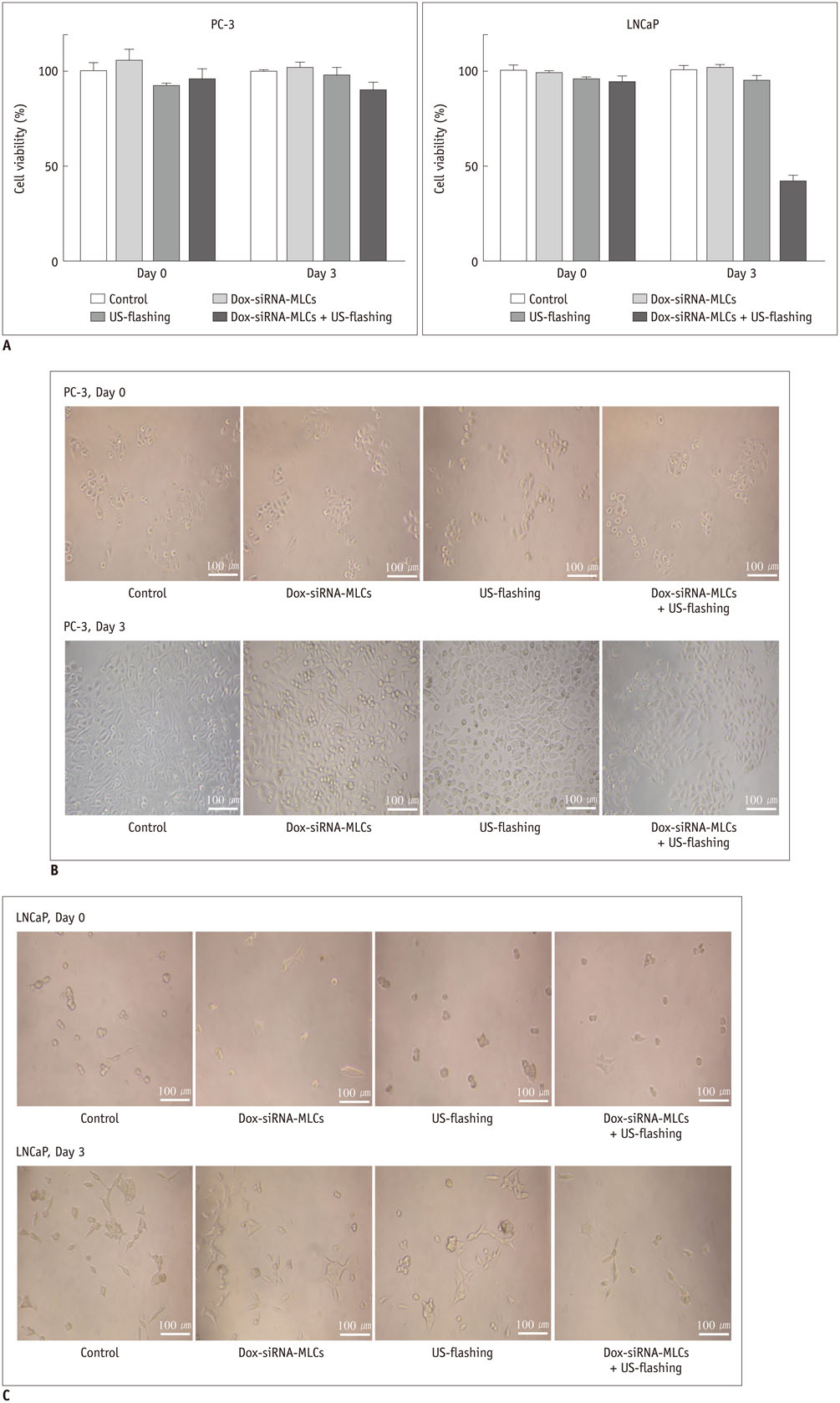
Microbubble-liposome complexes conjugated with anti-human epidermal growth factor receptor type 2 (Her2) antibodies were developed to target human prostate cancer cell lines PC-3 and LNCaP. Intracellular delivery of MLC was observed by confocal microscopy. We loaded MLC with survivin-targeted small interfering RNA (siRNA) and doxorubicin, and delivered it into prostate cancer cells. The release of these agents was facilitated by ultrasound application. Cell viability was analyzed by MTT assay after the delivery of siRNA and doxorubicin. Survivin-targeted siRNA loaded MLC was delivered into the xenograft mouse tumor model. Western blotting was performed to quantify the expression of survivin in vivo.
RESULTS
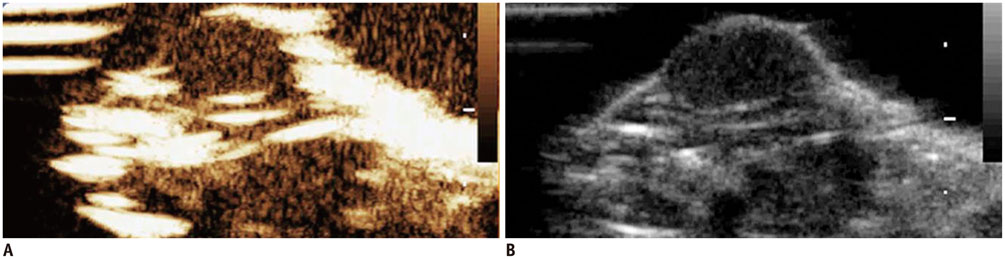
Confocal microscopy demonstrated substantial intracellular uptake of MLCs in LNCaP, which expresses higher levels of Her2 than PC-3. The viability of LNCaP cells was significantly reduced after the delivery of MLCs loaded with siRNA and doxorubicin (85.0 ± 2.9%), which was further potentiated by application of ultrasound (55.0 ± 3.5%, p = 0.009). Survivin expression was suppressed in vivo in LNCaP tumor xenograft model following the ultrasound and MLC-guided delivery of siRNA (77.4 ± 4.90% to 36.7 ± 1.34%, p = 0.027).
CONCLUSION
Microbubble-liposome complex can effectively target prostate cancer cells, enabling intracellular delivery of the treatment agents with the use of ultrasound. Ultrasound and MLC-mediated delivery of survivin-targeted siRNA and doxorubicin can induce prostate cell apoptosis and block survivin expression in vitro and in vivo.
MeSH Terms
-
Animals
Antibiotics, Antineoplastic/*therapeutic use/toxicity
Cell Line, Tumor
Cell Survival/drug effects
Disease Models, Animal
Doxorubicin/*therapeutic use/toxicity
Humans
Inhibitor of Apoptosis Proteins/antagonists & inhibitors/genetics/metabolism
Liposomes/chemistry
Male
Mice
*Microbubbles
Microscopy, Confocal
Prostatic Neoplasms/*drug therapy/pathology
RNA Interference
RNA, Small Interfering/genetics/*metabolism/therapeutic use
Transfection
Transplantation, Heterologous
Ultrasonography
Antibiotics, Antineoplastic
Inhibitor of Apoptosis Proteins
Liposomes
RNA, Small Interfering
Doxorubicin
Figure
Reference
-
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.2. Kawasaki H, Taira K, Morris KV. siRNA induced transcriptional gene silencing in mammalian cells. Cell Cycle. 2005; 4:442–448.3. Barik S. Silence of the transcripts: RNA interference in medicine. J Mol Med (Berl). 2005; 83:764–773.4. Hasan W, Chu K, Gullapalli A, Dunn SS, Enlow EM, Luft JC, et al. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett. 2012; 12:287–292.5. Becker AL, Orlotti NI, Folini M, Cavalieri F, Zelikin AN, Johnston AP, et al. Redox-active polymer microcapsules for the delivery of a survivin-specific siRNA in prostate cancer cells. ACS Nano. 2011; 5:1335–1344.6. Xue HY, Wong HL. Tailoring nanostructured solid-lipid carriers for time-controlled intracellular siRNA kinetics to sustain RNAi-mediated chemosensitization. Biomaterials. 2011; 32:2662–2672.7. Yoon YI, Kwon YS, Cho HS, Heo SH, Park KS, Park SG, et al. Ultrasound-mediated gene and drug delivery using a microbubble-liposome particle system. Theranostics. 2014; 4:1133–1144.8. Wang X, Liang HD, Dong B, Lu QL, Blomley MJ. Gene transfer with microbubble ultrasound and plasmid DNA into skeletal muscle of mice: comparison between commercially available microbubble contrast agents. Radiology. 2005; 237:224–229.9. Blomley MJ, Cooke JC, Unger EC, Monaghan MJ, Cosgrove DO. Microbubble contrast agents: a new era in ultrasound. BMJ. 2001; 322:1222–1225.10. Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev. 2008; 60:1153–1166.11. Wu Y, Unger EC, McCreery TP, Sweitzer RH, Shen D, Wu G, et al. Binding and lysing of blood clots using MRX-408. Invest Radiol. 1998; 33:880–885.12. Malmberg J, Tolmachev V, Orlova A. Imaging agents for in vivo molecular profiling of disseminated prostate cancer: cellular processing of [(111)In]-labeled CHX-A"DTPA-trastuzumab and anti-HER2 ABY-025 Affibody in prostate cancer cell lines. Exp Ther Med. 2011; 2:523–528.13. Ling YX, Tao J, Fang SF, Hui Z, Fang QR. Downregulation of Id1 by small interfering RNA in prostate cancer PC3 cells in vivo and in vitro. Eur J Cancer Prev. 2011; 20:9–17.14. Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996; 28:251–265.15. Petrioli R, Paolelli L, Francini E, Manganelli A, Salvestrini F, Francini G. Weekly docetaxel and epirubicin in treatment of advanced hormone-refractory prostate cancer. Urology. 2007; 69:142–146.16. Paduano F, Villa R, Pennati M, Folini M, Binda M, Daidone MG, et al. Silencing of survivin gene by small interfering RNAs produces supra-additive growth suppression in combination with 17-allylamino-17-demethoxygeldanamycin in human prostate cancer cells. Mol Cancer Ther. 2006; 5:179–186.17. Koike H, Morikawa Y, Sekine Y, Matsui H, Shibata Y, Suzuki K. Survivin is associated with cell proliferation and has a role in 1a,25-dihydroxyvitamin D3 induced cell growth inhibition in prostate cancer. J Urol. 2011; 185:1497–1503.18. McEleny KR, Watson RW, Coffey RN, O'Neill AJ, Fitzpatrick JM. Inhibitors of apoptosis proteins in prostate cancer cell lines. Prostate. 2002; 51:133–140.19. Zhang M, Latham DE, Delaney MA, Chakravarti A. Survivin mediates resistance to antiandrogen therapy in prostate cancer. Oncogene. 2005; 24:2474–2482.20. Zhang M, Coen JJ, Suzuki Y, Siedow MR, Niemierko A, Khor LY, et al. Survivin is a potential mediator of prostate cancer metastasis. Int J Radiat Oncol Biol Phys. 2010; 78:1095–1103.21. Rahman KM, Banerjee S, Ali S, Ahmad A, Wang Z, Kong D, et al. 3,3'-Diindolylmethane enhances taxotere-induced apoptosis in hormone-refractory prostate cancer cells through survivin down-regulation. Cancer Res. 2009; 69:4468–4475.22. Shen J, Liu J, Long Y, Miao Y, Su M, Zhang Q, et al. Knockdown of survivin expression by siRNAs enhances chemosensitivity of prostate cancer cells and attenuates its tumorigenicity. Acta Biochim Biophys Sin (Shanghai). 2009; 41:223–230.23. Skyba DM, Price RJ, Linka AZ, Skalak TC, Kaul S. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation. 1998; 98:290–293.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrasound-guided drug delivery in cancer
- Enhanced Chemotherapeutic Drug Delivery to Tumor Tissue by High Intensity Focused Ultrasound
- Pain during Transrectal Ultrasound-Guided Prostate Biopsy and the Role of Periprostatic Nerve Block: What Radiologists Should Know
- Medical imaging of prostate cancer
- Questioning the evidence behind the Saturation Model for testosterone replacement therapy in prostate cancer