Ann Pediatr Endocrinol Metab.
2019 Mar;24(1):64-67. 10.6065/apem.2019.24.1.64.
Infantile hypercalcemia with novel compound heterozygous mutation in SLC34A1 encoding renal sodium-phosphate cotransporter 2a: a case report
- Affiliations
-
- 1Department of Pediatrics, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea.
- KMID: 2447379
- DOI: http://doi.org/10.6065/apem.2019.24.1.64
Abstract
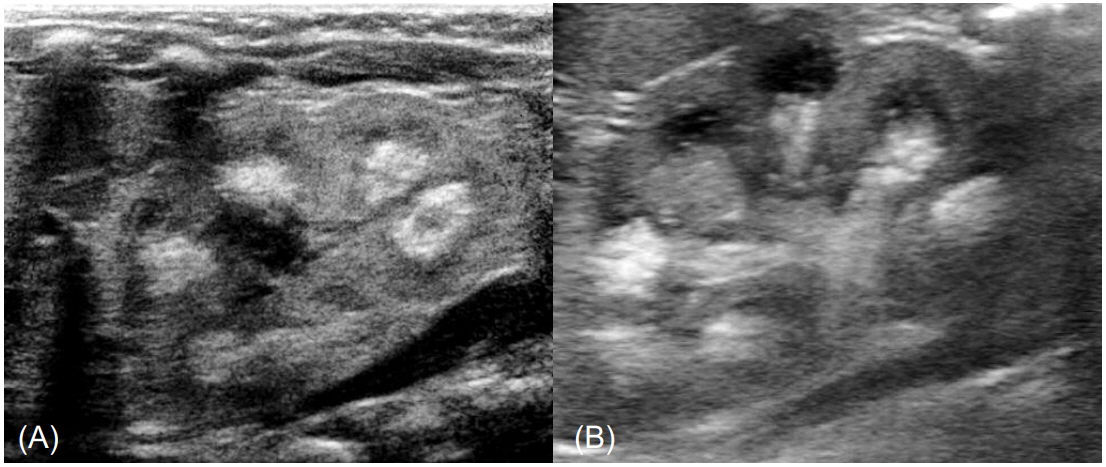
- Idiopathic infantile hypercalcemia is characterized by hypercalcemia, dehydration, vomiting, and failure to thrive, and it is due to mutations in 24-hydroxylase (CYP24A1). Recently, mutations in sodium-phosphate cotransporter (SLC34A1) expressed in the kidney were discovered as an additional cause of idiopathic infantile hypercalcemia. This report describes a female infant admitted for evaluation of nephrocalcinosis. She presented with hypercalcemia, hypercalciuria, low intact parathyroid hormone level, and high 1,25-dihydroxyvitamin D3 level. Exome sequencing identified novel compound heterozygous mutations in SLC34A1 (c.1337G>A, c.1483C>T). The patient was treated with fluids for hydration, furosemide, a corticosteroid, and restriction of calcium/vitamin D intake. At the age of 7 months, the patient's calcium level was within the normal range, and hypercalciuria waxed and waned. Renal echogenicity improved on the follow-up ultrasonogram, and developmental delay was not noted. In cases of hypercalcemia with subsequent hypercalciuria, DNA analysis for SLC34A1 gene mutations and CYP24A1 gene mutations should be performed. Further studies are required to obtain long-term data on hypercalciuria and nephrocalcinosis.
Keyword
MeSH Terms
-
Calcitriol
Calcium
Dehydration
DNA
Exome
Failure to Thrive
Female
Follow-Up Studies
Furosemide
Humans
Hypercalcemia*
Hypercalciuria
Hypophosphatemia
Infant
Kidney
Nephrocalcinosis
Parathyroid Hormone
Reference Values
Sodium-Phosphate Cotransporter Proteins*
Ultrasonography
Vitamin D
Vitamin D3 24-Hydroxylase
Vomiting
Calcitriol
Calcium
DNA
Furosemide
Parathyroid Hormone
Sodium-Phosphate Cotransporter Proteins
Vitamin D
Vitamin D3 24-Hydroxylase
Figure
Cited by 1 articles
-
Idiopathic infantile hypercalcemia with severe nephrocalcinosis, associated with
CYP24A1 mutations: a case report
Jeesun Yoo, Hee Gyung Kang, Yo Han Ahn
Child Kidney Dis. 2022;26(1):63-67. doi: 10.3339/ckd.22.027.
Reference
-
References
1. Lightwood R, Stapleton T. Idiopathic hypercalcaemia in infants. Lancet. 1953; 265:255–6.2. Koltin D, Rachmiel M, Wong BY, Cole DE, Harvey E, Sochett E. Mild infantile hypercalcemia: diagnostic tests and outcomes. J Pediatr. 2011; 159:215–21. e1.
Article3. Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med. 2011; 365:410–21.
Article4. Schlingmann KP, Ruminska J, Kaufmann M, Dursun I, Patti M, Kranz B, et al. Autosomal-recessive mutations in SLC34A1 encoding sodium-phosphate cotransporter 2A cause idiopathic infantile hypercalcemia. J Am Soc Nephrol. 2016; 27:604–14.
Article5. Rajagopal A, Braslavsky D, Lu JT, Kleppe S, Clement F, Cassinelli H, et al. Exome sequencing identifies a novel homozygous mutation in the phosphate transporter SLC34A1 in hypophosphatemia and nephrocalcinosis. J Clin Endocrinol Metab. 2014; 99:E2451–6.
Article6. Prie D, Friedlander G. Genetic disorders of renal phosphate transport. N Engl J Med. 2010; 362:2399–409.
Article7. Wagner CA, Hernando N, Forster IC, Biber J. The SLC34 family of sodium-dependent phosphate transporters. Pflugers Arch. 2014; 466:139–53.
Article8. Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005; 146:5358–64.
Article9. Wang W, Kwon TH, Li C, Frokiaer J, Knepper MA, Nielsen S. Reduced expression of Na-K-2Cl cotransporter in medullary TAL in vitamin D-induced hypercalcemia in rats. Am J Physiol Renal Physiol. 2002; 282:F34–44.
Article10. Ronnefarth G, Misselwitz J. Nephrocalcinosis in children: a retrospective survey. Members of the Arbeitsgemeinschaft fur padiatrische Nephrologie. Pediatr Nephrol. 2000; 14:1016–21.11. Halbritter J, Baum M, Hynes AM, Rice SJ, Thwaites DT, Gucev ZS, et al. Fourteen monogenic genes account for 15% of nephrolithiasis/nephrocalcinosis. J Am Soc Nephrol. 2015; 26:543–51.
Article12. Lapointe JY, Tessier J, Paquette Y, Wallendorff B, Coady MJ, Pichette V, et al. NPT2a gene variation in calcium nephrolithiasis with renal phosphate leak. Kidney Int. 2006; 69:2261–7.
Article13. Rodd C, Goodyer P. Hypercalcemia of the newborn: etiology, evaluation, and management. Pediatr Nephrol. 1999; 13:542–7.
Article14. Fencl F, Blahova K, Schlingmann KP, Konrad M, Seeman T. Severe hypercalcemic crisis in an infant with idiopathic infantile hypercalcemia caused by mutation in CYP24A1 gene. Eur J Pediatr. 2013; 172:45–9.
Article15. Marks BE, Doyle DA. Idiopathic infantile hypercalcemia: case report and review of the literature. J Pediatr Endocrinol Metab. 2016; 29:127–32.
Article16. Habbig S, Beck BB, Hoppe B. Nephrocalcinosis and urolithiasis in children. Kidney Int. 2011; 80:1278–91.
Article17. Odvina CV, Preminger GM, Lindberg JS, Moe OW, Pak CY. Long-term combined treatment with thiazide and potassium citrate in nephrolithiasis does not lead to hypokalemia or hypochloremic metabolic alkalosis. Kidney Int. 2003; 63:240–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Idiopathic infantile hypercalcemia with severe nephrocalcinosis, associated with CYP24A1 mutations: a case report
- A Case of Acute Phosphate Nephropathy after Sodium Phosphate Preparation
- A Case of Idiopathic Infantile Hypercalcemia Treated with Intravenous Pamidronate Infusion
- Multiple Endocrine Neoplasia 2A Detected by Peptic Ulcer Perforation with Hypercalcemia
- Kidney and Phosphate Metabolism