Yonsei Med J.
2005 Dec;46(6):835-842.
Combined Treatment of an Intratumoral Injection of Dendritic Cells and Systemic Chemotherapy (Paclitaxel) for Murine Fibrosarcoma
- Affiliations
-
- 1Department of Dermatology, Inha University College of Medicine, Inchon, Korea.
- 2Department of Hematology-Oncology, Inha University College of Medicine, Inchon, Korea.
- 3Clinical Research Center, Inha University College of Medicine, Inchon, Korea.
- 4Department of Dermatology, Yonsei University College of Medicine, Seoul, Korea.
Abstract
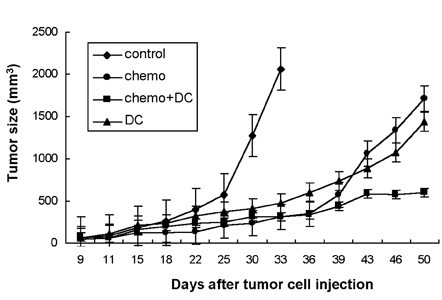
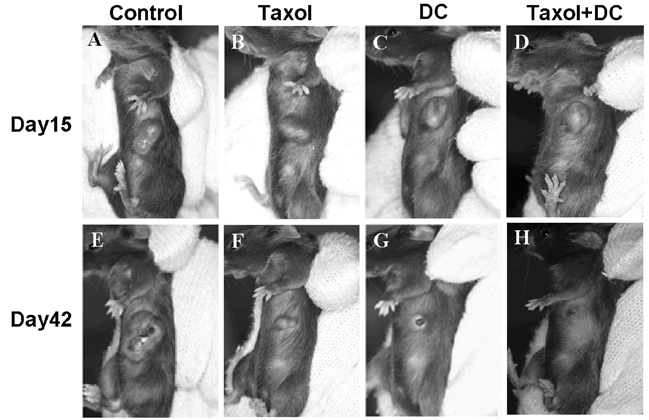
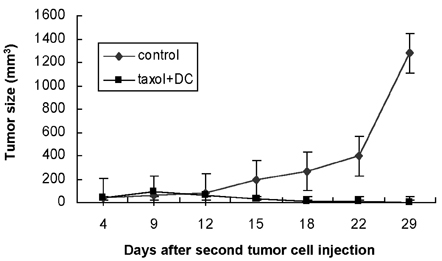
- A novel combined treatment of conventional chemotherapy with an intratumoral injection of syngeneic dendritic cells (DCs) has emerged as a potent cancer treatment strategy. In this study, we evaluated the synergistic effect of an intraperitoneal (i.p.) injection of a chemotherapeutic drug, paclitaxel, and an intratumoral (i.t.) injection of syngeneic bone marrow- derived DCs for the treatment of pre-existing fibrosarcoma. Subcutaneous tumors were established using MCA102 fibrosarcoma cells in syngeneic C57BL/6 mice. The results demonstrated that the combined treatment of paclitaxel chemotherapy and the injection of DCs led to complete tumor regression, in contrast to only partial eradication of the tumors with chemotherapy or DCs alone. Furthermore, the tumor-free mice were able to resist a repeat challenge with the same type of tumor. These findings suggest that a combination therapy of systemic chemotherapy along with the intratumoral administration of DCs is a potent treatment strategy for fibrosarcoma.
Keyword
MeSH Terms
-
Treatment Outcome
Transplantation, Isogeneic
Phenotype
Paclitaxel/administration & dosage/*therapeutic use
Mice
Injections, Intraperitoneal
Immunologic Memory
Fibrosarcoma/drug therapy/pathology/*therapy
Dendritic Cells/cytology/*transplantation
Combined Modality Therapy
Cells, Cultured
Cell Line, Tumor
Bone Marrow Cells/cytology
Antineoplastic Agents, Phytogenic/administration & dosage/*therapeutic use
Animals
Figure
Reference
-
1. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–252.2. Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991. 9:271–296.3. Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD Jr. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996. 183:283–287.4. Carbone FR, Kurts C, Bennett SR, Miller JF, Heath WR. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol Today. 1998. 19:368–373.5. Grabbe S, Beissert S, Schwarz T, Granstein RD. Dendritic cells as initiators of tumor immune responses: a possible strategy for tumor immunotherapy? Immunol Today. 1995. 16:117–121.6. Gabrilovich DI, Nadaf S, Corak J, Berzofsky JA, Carbone DP. Dendritic cells in antitumor immune responses. II. Dendritic cells grown from bone marrow precursors, but not mature DC from tumor-bearing mice, are effective antigen carriers in the therapy of established tumors. Cell Immunol. 1996. 170:111–119.7. Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998. 4:328–332.8. Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996. 184:465–472.9. Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD Jr. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996. 2:1122–1128.10. Gong J, Avigan D, Chen D, Wu Z, Koido S, Kashiwaba M, Kufe D. Activation of antitumor cytotoxic T lymphocytes by fusions of human dendritic cells and breast carcinoma cells. Proc Natl Acad Sci USA. 2000. 97:2715–2718.11. Song SY, Kim HS. Strategies to improve dendritic cell-based immunotherapy against cancer. Yonsei Med J. 2004. 45:Suppl. 48–52.12. Tong Y, Song W, Crystal RG. Combined intratumoral injection of bone marrow-derived dendritic cells and systemic chemotherapy to treat pre-existing murine tumors. Cancer Res. 2001. 61:7530–7535.13. Inoue N, Yamasaki S, Kondo K, Kan T, Furumoto K, Imamura M. Dendritic cells coinjected with tumor cells treated with an anticancer drug to induce tumor rejection. Surg Today. 2003. 33:269–276.14. Shin JY, Lee SK, Kang CD, Chung JS, Lee EY, Seo SY, et al. Antitumor effect of intratumoral administration of dendritic cell combination with vincristine chemotherapy in a murine fibrosarcoma model. Histol Histopathol. 2003. 18:435–447.15. Yu B, Kusmartsev S, Cheng F, Paolini M, Nefedova Y, Sotomayor E, et al. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clin Cancer Res. 2003. 9:285–294.16. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979. 277:665–667.17. Joo HG. Altered maturation of dendritic cells by taxol, an anticancer drug. J Vet Sci. 2003. 4:229–234.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined Treatment of Murine Fibrosarcoma with Chemotherapy (Paclitaxel), Radiotherapy, and Intratumoral Injection of Dendritic Cells
- Intratumoral Administration of Dendritic Cells Combined with Hyperthermia Induces Both Local and Systemic Antitumor Effect in Murine Tumor Models
- Intratumoral Administration of Secondary Lymphoid Chemokine and Unmethylated Cytosine-phosphorothioate-guanine Oligodeoxynucleotide Synergistically Inhibits Tumor Growth in Vivo
- Comparative Effects of Paclitaxel and Nitric Oxide on Superficial Murine Bladder Tumor Cells
- Enhancement of Tumor Radioresponse by Combined Chemotherapy in Murine Hepatocarcinoma