Ann Dermatol.
2014 Feb;26(1):53-60. 10.5021/ad.2014.26.1.53.
Combined Treatment of Murine Fibrosarcoma with Chemotherapy (Paclitaxel), Radiotherapy, and Intratumoral Injection of Dendritic Cells
- Affiliations
-
- 1Department of Dermatology, Inha University School of Medicine, Incheon, Korea. garden@inha.ac.kr
- 2Clinical Research Center, Inha University School of Medicine, Incheon, Korea.
- 3Saybeauty Clinic, Incheon, Korea.
- 4Department of Radiation Oncology, Inha University School of Medicine, Incheon, Korea.
- 5Department of Hemato-Oncology, Inha University School of Medicine, Incheon, Korea.
- KMID: 2265697
- DOI: http://doi.org/10.5021/ad.2014.26.1.53
Abstract
- BACKGROUND
New antitumor therapeutic strategies aim to combine different approaches that are able to induce tumor-specific effector and memory T cell responses that might control tumor growth. Dendritic cells (DCs) have the capacity to induce antigen-specific cytotoxic T lymphocytes. We have previously shown that the combined treatment of paclitaxel chemotherapy (Chemo) and injection of DCs led to complete tumor regression.
OBJECTIVE
The goal of this study was to evaluate synergistic antitumor effect of a triple combination treatment comprising radiotherapy, paclitaxel Chemo and intratumoral injection of syngeneic bone marrow-derived DCs on murine fibrosarcoma, compared to other single or double combination treatments.
METHODS
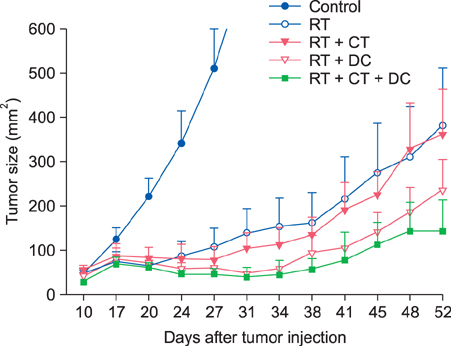
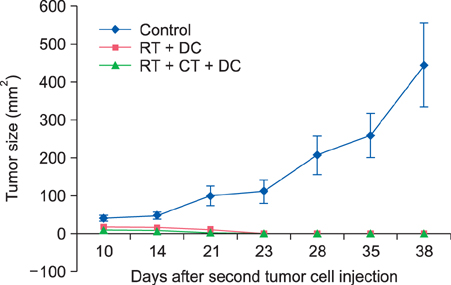
For the murine fibrosarcoma model, naive C57BL/6 mice were inoculated intradermally with 2x10(3) MCA102 cells in the right upper flank. Mice were assigned to five groups (untreatedcontrol, RT alone, RT+Chemo, RT+DC, and RT+Chemo+DC), with eight mice in each group. In vitro cytotoxicity assays were performed to assess the immune activity. The persistence of tumor-specific immunity was determined by second tumor challenge in mice with complete tumor regression.
RESULTS
The triple combination treatment showed a significantly enhanced therapeutic efficacy by decreasing tumor size and inducing complete tumor regression, resulting in a cure of 50% of mice. The results of in vitro cytotoxicity assays and the second tumor challenge experiment strongly indicated the induction of a tumor-specific cytotoxic T lymphocyte response and acquisition of prolonged tumor immunity.
CONCLUSION
These findings suggest that the triple combination treatment can be a promising strategy for the treatment of murine fibrosarcoma.
MeSH Terms
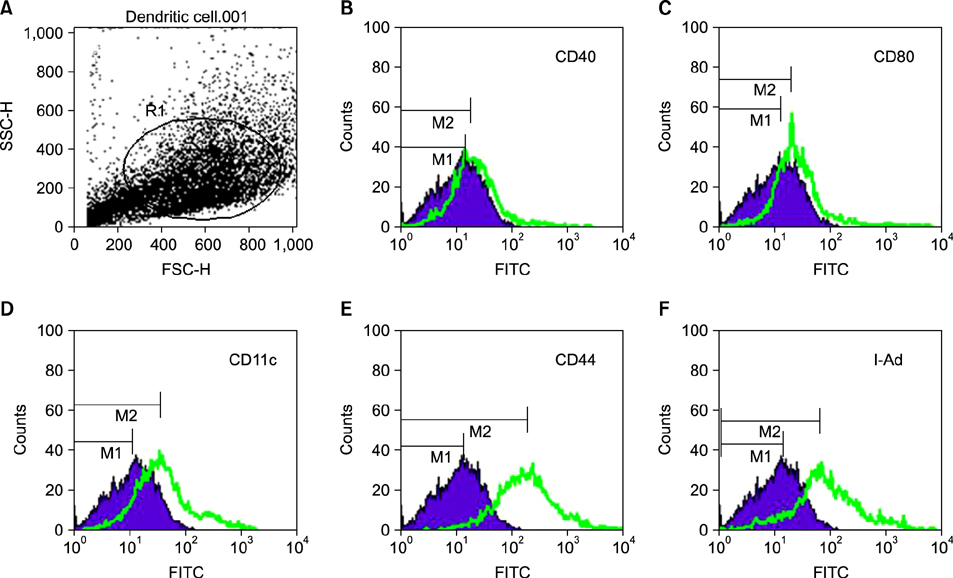
Figure
Reference
-
1. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998; 392:245–252.
Article2. Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991; 9:271–296.
Article3. Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD Jr. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996; 183:283–287.
Article4. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002; 3:991–998.
Article5. Restifo NP, Antony PA, Finkelstein SE, Leitner WW, Surman DP, Theoret MR, et al. Assumptions of the tumor 'escape' hypothesis. Semin Cancer Biol. 2002; 12:81–86.
Article6. Choi GS, Lee MH, Kim SK, Kim CS, Lee HS, Im MW, et al. Combined treatment of an intratumoral injection of dendritic cells and systemic chemotherapy (Paclitaxel) for murine fibrosarcoma. Yonsei Med J. 2005; 46:835–842.
Article7. Nowak AK, Robinson BW, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 2002; 62:2353–2358.8. Correale P, Cusi MG, Tsang KY, Del Vecchio MT, Marsili S, Placa ML, et al. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J Clin Oncol. 2005; 23:8950–8958.
Article9. Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007; 56:641–648.
Article10. Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007; 13:54–61.
Article11. Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des. 2002; 8:1765–1780.
Article12. Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003; 198:569–580.
Article13. Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005; 202:907–912.
Article14. Quarmby S, Kumar P, Kumar S. Radiation-induced normal tissue injury: role of adhesion molecules in leukocyteendothelial cell interactions. Int J Cancer. 1999; 82:385–395.
Article15. Ganss R, Ryschich E, Klar E, Arnold B, Hämmerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002; 62:1462–1470.16. Nikitina EY, Gabrilovich DI. Combination of gammairradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer. Int J Cancer. 2001; 94:825–833.
Article17. Spira AI, Ettinger DS. The use of chemotherapy in soft-tissue sarcomas. Oncologist. 2002; 7:348–359.
Article18. Berezhnaya NM, Vinnichuk UD, Belova OB, Baranovich VV. Antitumor action of lymphokin-activated cells of patients with soft tissue sarcomas and melanomas in dependence on expression of MHC classes I and II antigenes. Exp Oncol. 2006; 28:231–234.19. Kucera A, Pýcha K, Pajer P, Spísek R, Skába R. Dendritic cell-based immunotherapy induces transient clinical response in advanced rat fibrosarcoma - comparison with preventive anti-tumour vaccination. Folia Biol (Praha). 2009; 55:119–125.20. Shin JY, Lee SK, Kang CD, Chung JS, Lee EY, Seo SY, et al. Antitumor effect of intratumoral administration of dendritic cell combination with vincristine chemotherapy in a murine fibrosarcoma model. Histol Histopathol. 2003; 18:435–447.21. Finkelstein SE, Iclozan C, Bui MM, Cotter MJ, Ramakrishnan R, Ahmed J, et al. Combination of external beam radiotherapy (EBRT) with intratumoral injection of dendritic cells as neo-adjuvant treatment of high-risk soft tissue sarcoma patients. Int J Radiat Oncol Biol Phys. 2012; 82:924–932.
Article22. Derby E, Reddy V, Kopp W, Nelson E, Baseler M, Sayers T, et al. Three-color flow cytometric assay for the study of the mechanisms of cell-mediated cytotoxicity. Immunol Lett. 2001; 78:35–39.
Article23. Shah DA, Madden LV. Nonparametric analysis of ordinal data in designed factorial experiments. Phytopathology. 2004; 94:33–43.
Article24. Paulos CM, Kaiser A, Wrzesinski C, Hinrichs CS, Cassard L, Boni A, et al. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007; 13:5280–5289.
Article25. Fulda S, Los M, Friesen C, Debatin KM. Chemosensitivity of solid tumor cells in vitro is related to activation of the CD95 system. Int J Cancer. 1998; 76:105–114.
Article26. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007; 13:1050–1059.
Article27. Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000; 191:423–434.28. Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998; 392:86–89.
Article29. Tsujitani S, Kakeji Y, Watanabe A, Kohnoe S, Maehara Y, Sugimaghi K. Infiltration of S-100 protein positive dendritic cells and peritoneal recurrence in advanced gastric cancer. Int Surg. 1992; 77:238–241.30. Machlenkin A, Goldberger O, Tirosh B, Paz A, Volovitz I, Bar-Haim E, et al. Combined dendritic cell cryotherapy of tumor induces systemic antimetastatic immunity. Clin Cancer Res. 2005; 11:4955–4961.
Article31. Candido KA, Shimizu K, McLaughlin JC, Kunkel R, Fuller JA, Redman BG, et al. Local administration of dendritic cells inhibits established breast tumor growth: implications for apoptosis-inducing agents. Cancer Res. 2001; 61:228–236.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined Treatment of an Intratumoral Injection of Dendritic Cells and Systemic Chemotherapy (Paclitaxel) for Murine Fibrosarcoma
- Intratumoral Administration of Secondary Lymphoid Chemokine and Unmethylated Cytosine-phosphorothioate-guanine Oligodeoxynucleotide Synergistically Inhibits Tumor Growth in Vivo
- Intratumoral Administration of Dendritic Cells Combined with Hyperthermia Induces Both Local and Systemic Antitumor Effect in Murine Tumor Models
- Successful Treatment of Infantile Fibrosarcoma Spinal Metastasis by Chemotherapy and Stereotactic Hypofractionated Radiotherapy
- Paclitaxel and Cisplatin with Concurrent Radiotherapy for Stage III Non-Small Cell Lung Cancer