Cancer Res Treat.
2018 Jul;50(3):1023-1038. 10.4143/crt.2017.085.
Everolimus Plus Ku0063794 Regimen Promotes Anticancer Effects against Hepatocellular Carcinoma Cells through the Paradoxical Inhibition of Autophagy
- Affiliations
-
- 1Department of Surgery, Daejeon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Daejeon, Korea.
- 2Department of Surgery, Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu, Korea.
- 3Department of Surgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. sayjunekim@gmail.com
- 4The Catholic Central Laboratory of Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2417890
- DOI: http://doi.org/10.4143/crt.2017.085
Abstract
- PURPOSE
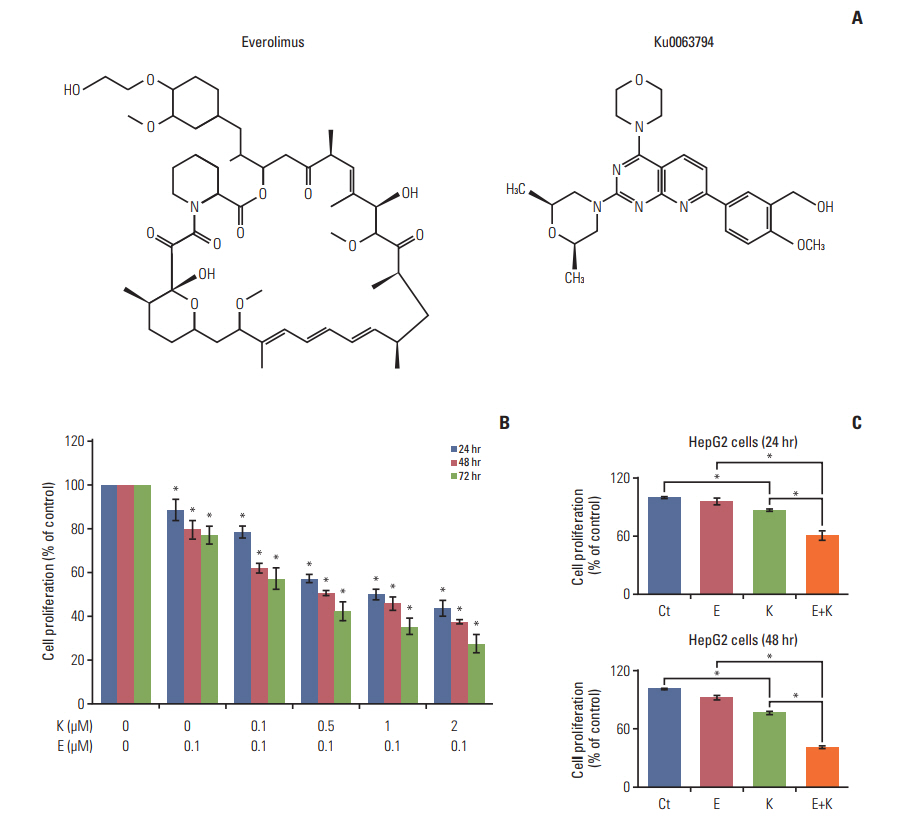
Everolimus only inhibits mammalian target of rapamycin complex 1 (mTORC1), whereas Ku0063794 inhibits both mTORC1 and mTORC2. Although they have similar anticancer effects, their combination has a synergistic effect against hepatocellular carcinoma (HCC) cells. We aimed to determine the mechanism underlying the synergistic effects of everolimus and Ku0063794 associated with autophagy in HCC cells.
MATERIALS AND METHODS
We compared the effects of everolimus and Ku0063794, individually or in combination, on both the in vitro and in vivo models of HCCs.
RESULTS
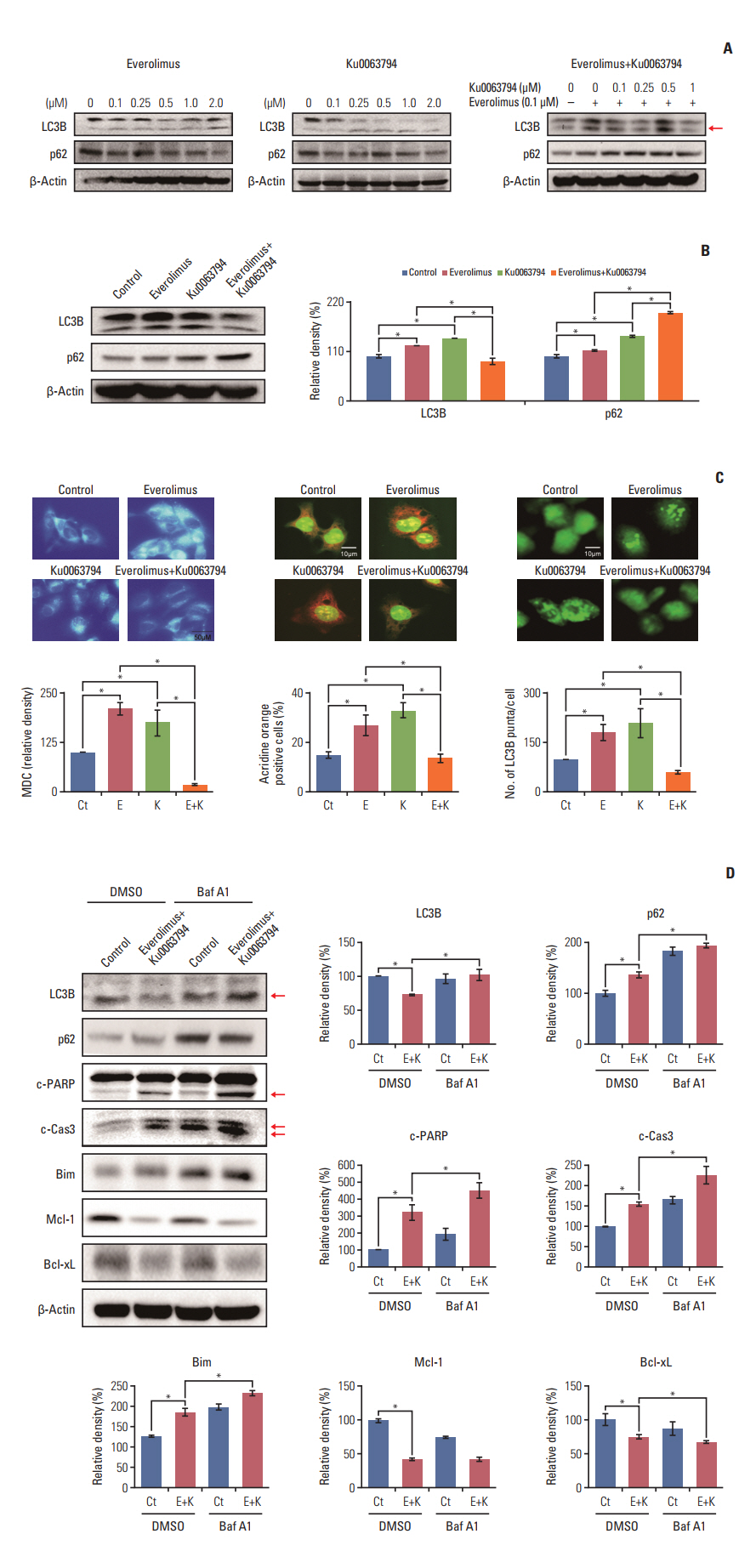
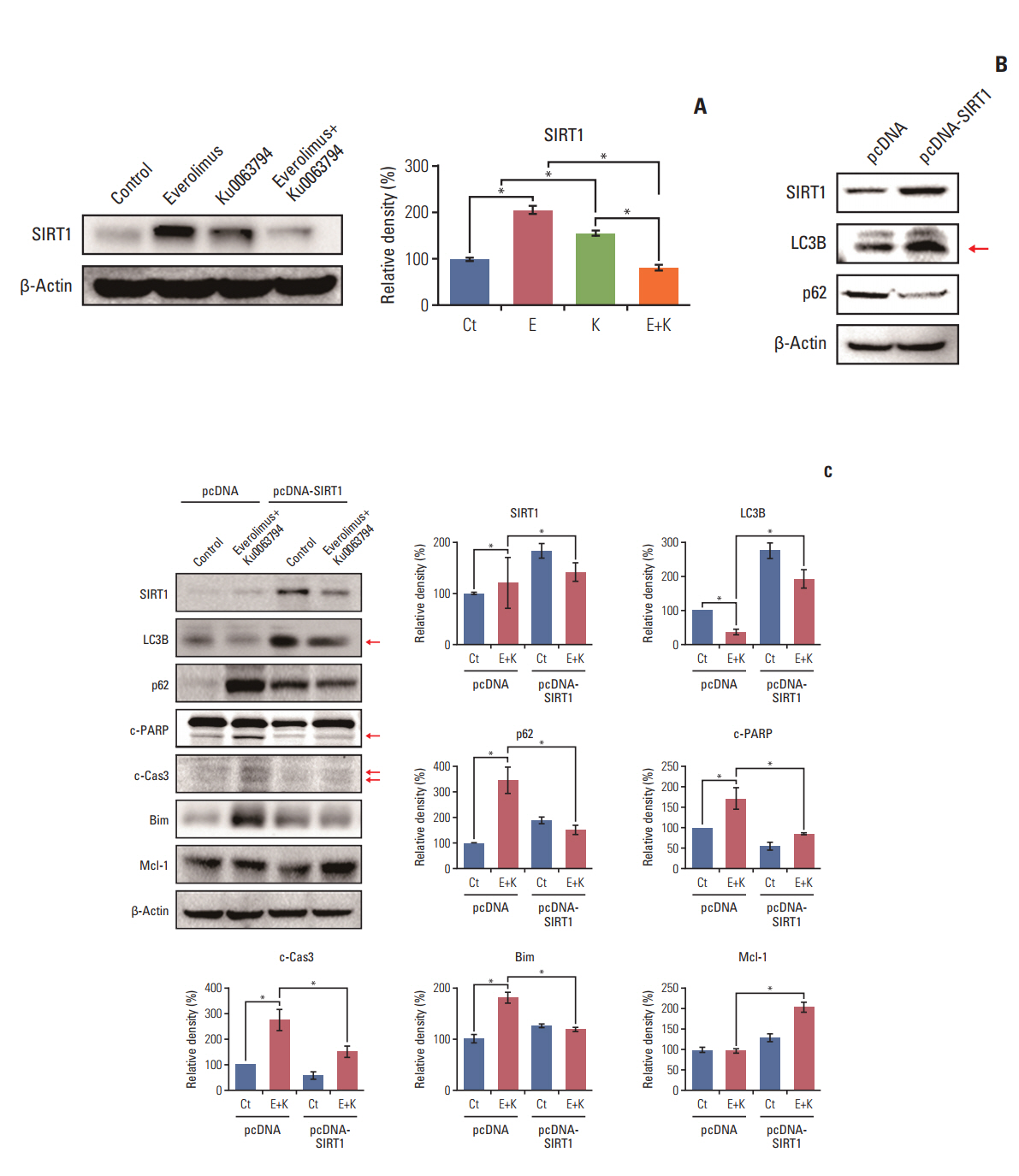
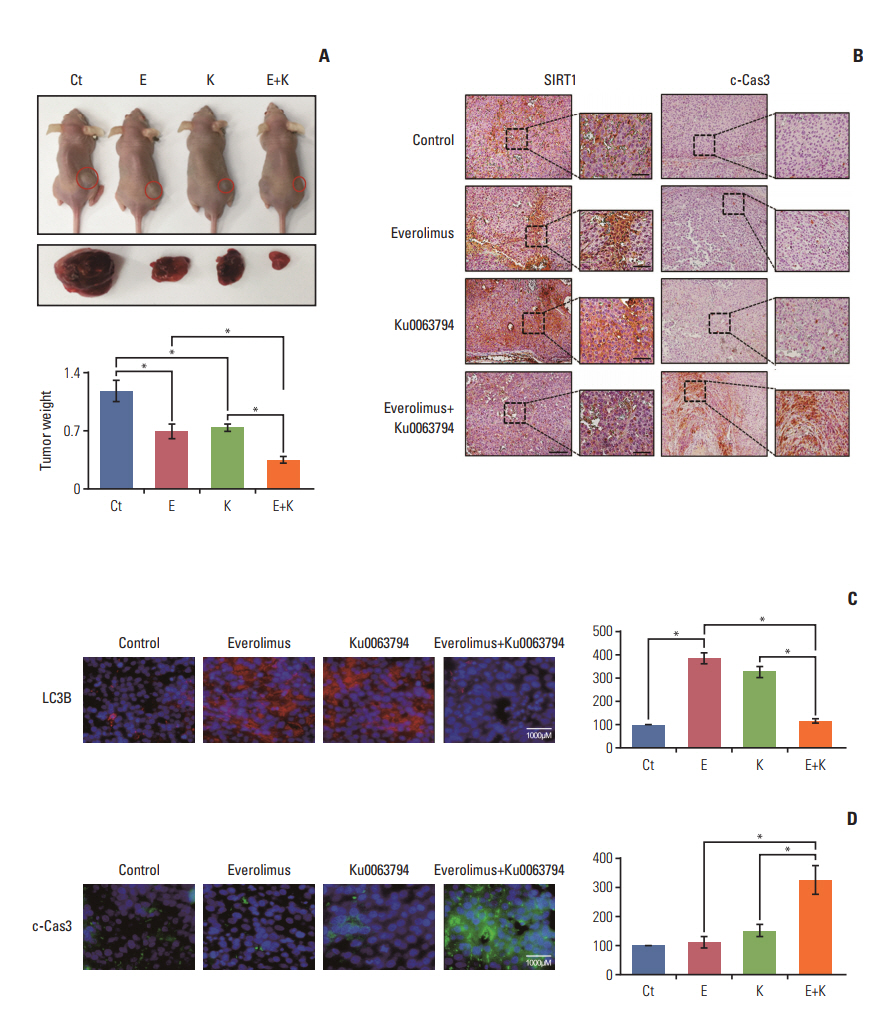
HepG2 cells treated with both agents had significantly lower rates of cell proliferation and higher apoptosis than the individual monotherapies (p < 0.05). Autophagic studies consistently indicated that, unlike the monotherapies, the combination therapy significantly reduced autophagy (p < 0.05). Autophagic blockage directly promoted the pro-apoptotic effects of combination therapy, suggesting autophagy as the survival mechanism of HCC cells. Unlike the monotherapies, combination therapy showed the potential to inhibit sirtuin 1 (SIRT1), the positive regulator of autophagy. SIRT1 overexpression abrogated the autophagy-inhibiting and pro-apoptotic effects of combination therapy. In a nude mouse xenograft model, the shrinkage of tumors was more prominent in mice treated with combination therapy than in mice treated with the respective monotherapies (p < 0.05). The immunohistochemical and immunofluorescence stains of the tumor obtained from the xenograft model showed that combination therapy had the potential of reducing autophagy and promoting apoptosis.
CONCLUSION
The combination of everolimus and Ku0063794 potentiates anticancer effects on HCCs through a decrease in autophagy, which is prompted by SIRT1 downregulation.
MeSH Terms
-
Animals
Apoptosis
Autophagy*
Carcinoma, Hepatocellular*
Cell Proliferation
Coloring Agents
Down-Regulation
Everolimus*
Fluorescent Antibody Technique
Hep G2 Cells
Heterografts
In Vitro Techniques
Mice
Mice, Nude
Sirolimus
Sirtuin 1
TOR Serine-Threonine Kinases
Coloring Agents
Everolimus
Sirolimus
Sirtuin 1
TOR Serine-Threonine Kinases
Figure
Cited by 1 articles
-
Potentiation of the Anticancer Effects by Combining Docetaxel with Ku-0063794 against Triple-Negative Breast Cancer Cells
Ye-Won Jeon, Ok-Hee Kim, Jin Sun Shin, Ha Eun Hong, Cho Hee Kim, Say-June Kim
Cancer Res Treat. 2022;54(1):157-173. doi: 10.4143/crt.2020.1063.
Reference
-
References
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009; 27:1485–91.
Article2. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011; 365:1118–27.
Article3. Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008; 135:1972–83.
Article4. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012; 149:274–93.
Article5. Sparks CA, Guertin DA. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010; 29:3733–44.
Article6. Yamanaka K, Petrulionis M, Lin S, Gao C, Galli U, Richter S, et al. Therapeutic potential and adverse events of everolimus for treatment of hepatocellular carcinoma: systematic review and meta-analysis. Cancer Med. 2013; 2:862–71.7. Zhu AX, Abrams TA, Miksad R, Blaszkowsky LS, Meyerhardt JA, Zheng H, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011; 117:5094–102.
Article8. Kim JO, Kim KH, Song IS, Cheon KS, Kim OH, Lee SC, et al. Potentiation of the anticancer effects of everolimus using a dual mTORC1/2 inhibitor in hepatocellular carcinoma cells. Oncotarget. 2017; 8:2936–48.
Article9. Hamacher-Brady A, Brady NR, Gottlieb RA. The interplay between pro-death and pro-survival signaling pathways in myocardial ischemia/reperfusion injury: apoptosis meets autophagy. Cardiovasc Drugs Ther. 2006; 20:445–62.
Article10. Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010; 90:1383–435.
Article11. Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat Rev Mol Cell Biol. 2011; 13:7–12.
Article12. Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, Ohsumi Y. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol. 2000; 150:1507–13.
Article13. Mehrpour M, Esclatine A, Beau I, Codogno P. Autophagy in health and disease. 1. Regulation and significance of autophagy: an overview. Am J Physiol Cell Physiol. 2010; 298:C776–85.
Article14. Jeong JK, Moon MH, Lee YJ, Seol JW, Park SY. Autophagy induced by the class III histone deacetylase Sirt1 prevents prion peptide neurotoxicity. Neurobiol Aging. 2013; 34:146–56.
Article15. Lee JT, Gu W. SIRT1: regulator of p53 deacetylation. Genes Cancer. 2013; 4:112–7.
Article16. Baur JA. Biochemical effects of SIRT1 activators. Biochim Biophys Acta. 2010; 1804:1626–34.
Article17. Li X. SIRT1 and energy metabolism. Acta Biochim Biophys Sin (Shanghai). 2013; 45:51–60.
Article18. Fullgrabe J, Klionsky DJ, Joseph B. Histone post-translational modifications regulate autophagy flux and outcome. Autophagy. 2013; 9:1621–3.
Article19. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008; 105:3374–9.
Article20. Bradshaw-Pierce EL, Pitts TM, Kulikowski G, Selby H, Merz AL, Gustafson DL, et al. Utilization of quantitative in vivo pharmacology approaches to assess combination effects of everolimus and irinotecan in mouse xenograft models of colorectal cancer. PLoS One. 2013; 8:e58089.
Article21. Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005; 6:505–10.
Article22. Tang B, Dong X, Wei Z, Qiao H, Jiang H, Liu B, et al. Enhanced autophagy by everolimus contributes to the antirestenotic mechanisms in vascular smooth muscle cells. J Vasc Res. 2014; 51:259–68.
Article23. Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005; 6:298–305.
Article24. Ding ZB, Hui B, Shi YH, Zhou J, Peng YF, Gu CY, et al. Autophagy activation in hepatocellular carcinoma contributes to the tolerance of oxaliplatin via reactive oxygen species modulation. Clin Cancer Res. 2011; 17:6229–38.
Article25. Shimizu S, Takehara T, Hikita H, Kodama T, Tsunematsu H, Miyagi T, et al. Inhibition of autophagy potentiates the antitumor effect of the multikinase inhibitor sorafenib in hepatocellular carcinoma. Int J Cancer. 2012; 131:548–57.
Article26. Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007; 9:1102–9.
Article27. Ganley IG, Lam du H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009; 284:12297–305.
Article28. Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009; 20:1981–91.
Article29. Back JH, Rezvani HR, Zhu Y, Guyonnet-Duperat V, Athar M, Ratner D, et al. Cancer cell survival following DNA damagemediated premature senescence is regulated by mammalian target of rapamycin (mTOR)-dependent Inhibition of sirtuin 1. J Biol Chem. 2011; 286:19100–8.
Article30. Ma L, Dong W, Wang R, Li Y, Xu B, Zhang J, et al. Effect of caloric restriction on the SIRT1/mTOR signaling pathways in senile mice. Brain Res Bull. 2015; 116:67–72.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metformin Promotes Apoptosis but Suppresses Autophagy in Glucose-Deprived H4IIE Hepatocellular Carcinoma Cells
- Autophagy Inhibition Promotes Quercetin Induced Apoptosis in MG-63 Human Osteosarcoma cells
- Glutamine synthetase mediates sorafenib sensitivity in β-catenin-active hepatocellular carcinoma cells
- Transglutaminase 2 Promotes Autophagy by LC3 Induction through p53 Depletion in Cancer Cell
- Hepatitis B virus X Protein Promotes Liver Cancer Progression through Autophagy Induction in Response to TLR4 Stimulation