Diabetes Metab J.
2015 Dec;39(6):518-527. 10.4093/dmj.2015.39.6.518.
Metformin Promotes Apoptosis but Suppresses Autophagy in Glucose-Deprived H4IIE Hepatocellular Carcinoma Cells
- Affiliations
-
- 1Department of Histology and Institute of Medical Science, Jeju National University School of Medicine, Jeju, Korea. parkdb@jejunu.ac.kr
- KMID: 2174005
- DOI: http://doi.org/10.4093/dmj.2015.39.6.518
Abstract
- BACKGROUND
Metformin, a well-known anti-diabetic drug, has gained interest due to its association with the reduction of the prevalence of cancer in patients with type 2 diabetes and the anti-proliferative effect of metformin in several cancer cells. Here, we investigated the anti-proliferative effect of metformin with respect to apoptosis and autophagy in H4IIE hepatocellular carcinoma cells.
METHODS
H4IIE rat cells were treated with metformin in glucose-free medium for 24 hours and were then subjected to experiments examining the onset of apoptosis and/or autophagy as well as the related signaling pathways.
RESULTS
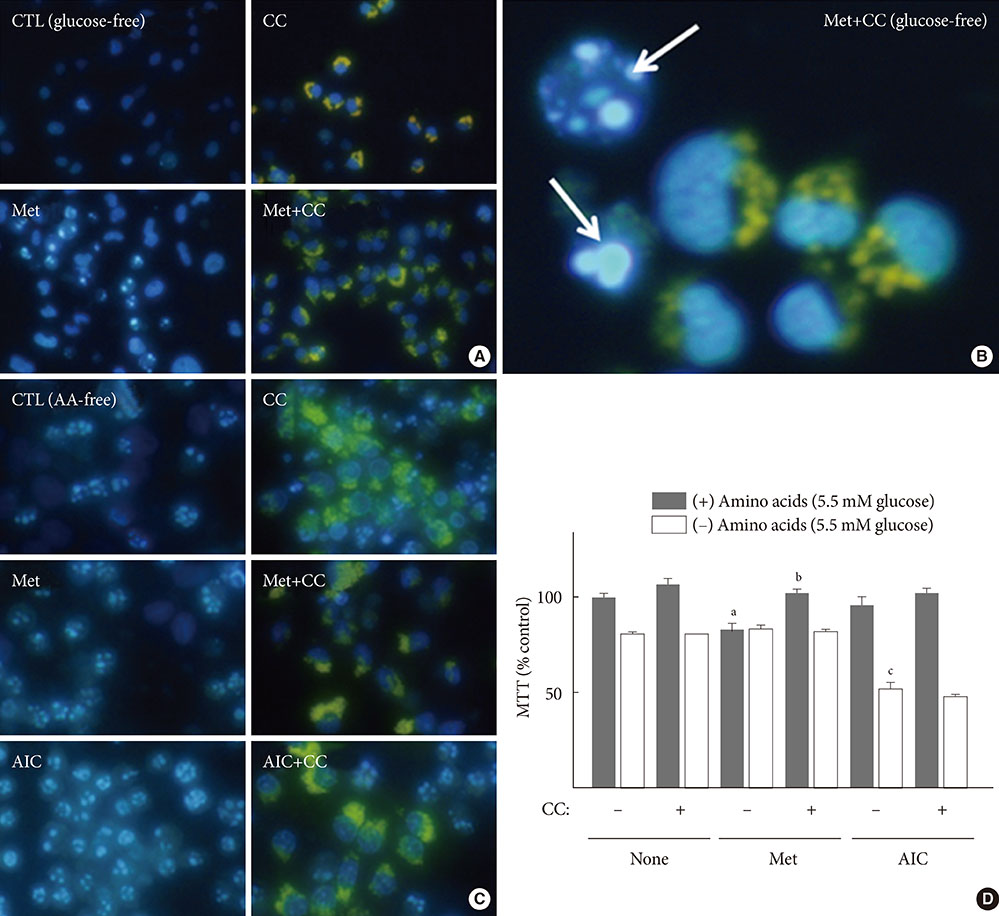
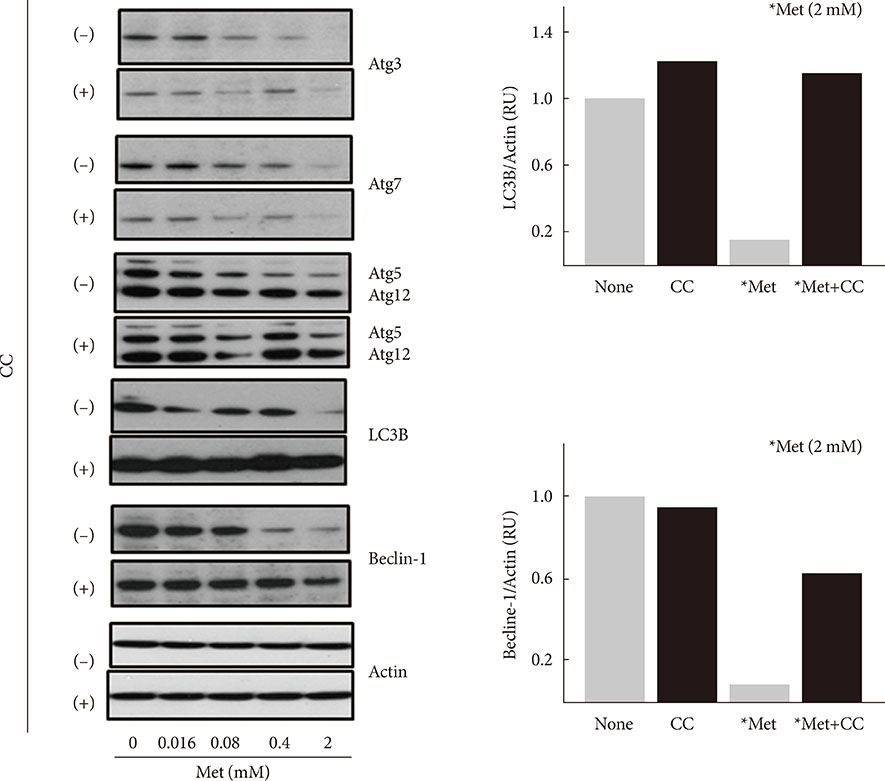
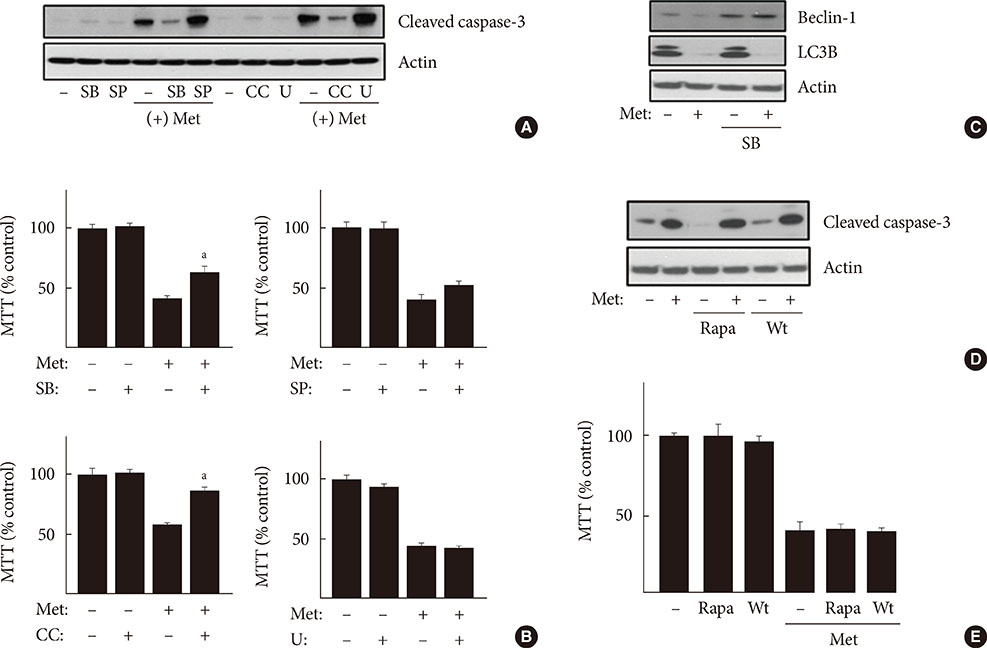
When H4IIE cells were incubated in glucose-free media for 24 hours, metformin and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) reduced the viability of cells. Inhibition of AMP-activated protein kinase (AMPK) by compound C significantly blocked cell death induced by metformin or AICAR. Pro-apoptotic events (nuclear condensation, hydrolysis of intact poly ADP ribose polymerase and caspase-3) were stimulated by metformin and then suppressed by compound C. Interestingly, the formation of acidic intracellular vesicles, a marker of autophagy, was stimulated by compound C. Although the deprivation of amino acids in culture media also induced apoptosis, neither metformin nor compound C affected cell viability. The expression levels of all of the autophagy-related proteins examined decreased with metformin, and two proteins (light chain 3 and beclin-1) were sensitive to compound C. Among the tested inhibitors against MAP kinases and phosphatidylinositol-3-kinase/mammalian target of rapamycin, SB202190 (against p38MAP kinase) significantly interrupted the effects of metformin.
CONCLUSION
Our data suggest that metformin induces apoptosis, but suppresses autophagy, in hepatocellular carcinoma cells via signaling pathways, including AMPK and p38 mitogen-activated protein kinase.
MeSH Terms
-
Amino Acids
AMP-Activated Protein Kinases
Animals
Apoptosis*
Autophagy*
Carcinoma, Hepatocellular*
Cell Death
Cell Survival
Culture Media
Humans
Hydrolysis
Metformin*
Phosphotransferases
Poly(ADP-ribose) Polymerases
Prevalence
Protein Kinases
Rats
Sirolimus
AMP-Activated Protein Kinases
Amino Acids
Culture Media
Metformin
Phosphotransferases
Poly(ADP-ribose) Polymerases
Protein Kinases
Sirolimus
Figure
Reference
-
1. Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012; 2:778–790.2. Park HK. Metformin and cancer in type 2 diabetes. Diabetes Metab J. 2013; 37:113–116.3. Rizos CV, Elisaf MS. Metformin and cancer. Eur J Pharmacol. 2013; 705:96–108.4. Ashinuma H, Takiguchi Y, Kitazono S, Kitazono-Saitoh M, Kitamura A, Chiba T, Tada Y, Kurosu K, Sakaida E, Sekine I, Tanabe N, Iwama A, Yokosuka O, Tatsumi K. Antiproliferative action of metformin in human lung cancer cell lines. Oncol Rep. 2012; 28:8–14.5. Kato K, Gong J, Iwama H, Kitanaka A, Tani J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y, Kobara H, Mori H, Himoto T, Okano K, Suzuki Y, Murao K, Masaki T. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol Cancer Ther. 2012; 11:549–560.6. Cantrell LA, Zhou C, Mendivil A, Malloy KM, Gehrig PA, Bae-Jump VL. Metformin is a potent inhibitor of endometrial cancer cell proliferation: implications for a novel treatment strategy. Gynecol Oncol. 2010; 116:92–98.7. Du Y, Zheng H, Wang J, Ren Y, Li M, Gong C, Xu F, Yang C. Metformin inhibits histone H2B monoubiquitination and downstream gene transcription in human breast cancer cells. Oncol Lett. 2014; 8:809–812.8. Bhalla K, Hwang BJ, Dewi RE, Twaddel W, Goloubeva OG, Wong KK, Saxena NK, Biswal S, Girnun GD. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev Res (Phila). 2012; 5:544–552.9. Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012; 13:251–262.10. Choi YK, Park KG. Metabolic roles of AMPK and metformin in cancer cells. Mol Cells. 2013; 36:279–287.11. Emami Riedmaier A, Fisel P, Nies AT, Schaeffeler E, Schwab M. Metformin and cancer: from the old medicine cabinet to pharmacological pitfalls and prospects. Trends Pharmacol Sci. 2013; 34:126–135.12. Quan W, Lee MS. Role of autophagy in the control of body metabolism. Endocrinol Metab (Seoul). 2013; 28:6–11.13. Gonzalez CD, Lee MS, Marchetti P, Pietropaolo M, Towns R, Vaccaro MI, Watada H, Wiley JW. The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy. 2011; 7:2–11.14. Lin F, Yan W, Wen T, Wu GY. Metformin induces apoptosis in hepatocellular carcinoma Huh-7 cells in vitro and its mechanism. Zhonghua Zhong Liu Za Zhi. 2013; 35:742–746.15. Miyoshi H, Kato K, Iwama H, Maeda E, Sakamoto T, Fujita K, Toyota Y, Tani J, Nomura T, Mimura S, Kobayashi M, Morishita A, Kobara H, Mori H, Yoneyama H, Deguchi A, Himoto T, Kurokohchi K, Okano K, Suzuki Y, Murao K, Masaki T. Effect of the anti-diabetic drug metformin in hepatocellular carcinoma in vitro and in vivo. Int J Oncol. 2014; 45:322–332.16. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.17. Jiang Y, Huang W, Wang J, Xu Z, He J, Lin X, Zhou Z, Zhang J. Metformin plays a dual role in MIN6 pancreatic beta cell function through AMPK-dependent autophagy. Int J Biol Sci. 2014; 10:268–277.18. Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, Zhang QQ, Gao L, Shi JQ, Zhang YD, Tan L. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014; 171:3146–3157.19. He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013; 62:1270–1281.20. Duan X, Ponomareva L, Veeranki S, Choubey D. IFI16 induction by glucose restriction in human fibroblasts contributes to autophagy through activation of the ATM/AMPK/p53 pathway. PLoS One. 2011; 6:e19532.21. Marambio P, Toro B, Sanhueza C, Troncoso R, Parra V, Verdejo H, Garcia L, Quiroga C, Munafo D, Diaz-Elizondo J, Bravo R, Gonzalez MJ, Diaz-Araya G, Pedrozo Z, Chiong M, Colombo MI, Lavandero S. Glucose deprivation causes oxidative stress and stimulates aggresome formation and autophagy in cultured cardiac myocytes. Biochim Biophys Acta. 2010; 1802:509–518.22. Carroll B, Korolchuk VI, Sarkar S. Amino acids and autophagy: cross-talk and co-operation to control cellular homeostasis. Amino Acids. 2015; 47:2065–2088.23. Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009; 16:1103–1123.24. Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009; 52:1766–1777.25. Taubes G. Cancer research. Cancer prevention with a diabetes pill? Science. 2012; 335:29.26. Chung HH, Moon JS, Yoon JS, Lee HW, Won KC. The relationship between metformin and cancer in patients with type 2 diabetes. Diabetes Metab J. 2013; 37:125–131.27. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998; 352:854–865.28. El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000; 275:223–228.29. Musi N. AMP-activated protein kinase and type 2 diabetes. Curr Med Chem. 2006; 13:583–589.30. Cheng J, Huang T, Li Y, Guo Y, Zhu Y, Wang Q, Tan X, Chen W, Zhang Y, Cheng W, Yamamoto T, Jing X, Huang J. AMP-activated protein kinase suppresses the in vitro and in vivo proliferation of hepatocellular carcinoma. PLoS One. 2014; 9:e93256.31. Xiao X, He Q, Lu C, Werle KD, Zhao RX, Chen J, Davis BC, Cui R, Liang J, Xu ZX. Metformin impairs the growth of liver kinase B1-intact cervical cancer cells. Gynecol Oncol. 2012; 127:249–255.32. Vytla VS, Ochs RS. Metformin increases mitochondrial energy formation in L6 muscle cell cultures. J Biol Chem. 2013; 288:20369–20377.33. Feng Y, Ke C, Tang Q, Dong H, Zheng X, Lin W, Ke J, Huang J, Yeung SC, Zhang H. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014; 5:e1088.34. Sanchez-Alvarez R, Martinez-Outschoorn UE, Lamb R, Hulit J, Howell A, Gandara R, Sartini M, Rubin E, Lisanti MP, Sotgia F. Mitochondrial dysfunction in breast cancer cells prevents tumor growth: understanding chemoprevention with metformin. Cell Cycle. 2013; 12:172–182.35. Din FV, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, Alessi DR, Dunlop MG. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012; 142:1504–1515.e3.36. Takahashi A, Kimura F, Yamanaka A, Takebayashi A, Kita N, Takahashi K, Murakami T. Metformin impairs growth of endometrial cancer cells via cell cycle arrest and concomitant autophagy and apoptosis. Cancer Cell Int. 2014; 14:53.37. Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, Le Marchand-Brustel Y, Giorgetti-Peraldi S, Cormont M, Bertolotto C, Deckert M, Auberger P, Tanti JF, Bost F. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010; 70:2465–2475.38. Queiroz EA, Puukila S, Eichler R, Sampaio SC, Forsyth HL, Lees SJ, Barbosa AM, Dekker RF, Fortes ZB, Khaper N. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS One. 2014; 9:e98207.39. Zhang Z, Zhang C, Ding Y, Zhao Q, Yang L, Ling J, Liu L, Ji H, Zhang Y. The activation of p38 and JNK by ROS, contribute to OLO-2-mediated intrinsic apoptosis in human hepatocellular carcinoma cells. Food Chem Toxicol. 2014; 63:38–47.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulatory Role of Autophagy in Globular Adiponectin-Induced Apoptosis in Cancer Cells
- Metformin and statins and their role in reducing hepatocellular carcinoma risk: Randomized trials are needed: Editorial on “Metformin and statins reduce hepatocellular carcinoma risk in chronic hepatitis C patients with failed antiviral therapy”
- Synergistic effect of metformin on sorafenib in in vitro study using hepatocellular carcinoma cell lines
- The Impact of Autophagy on the Cigarette Smoke Extract-Induced Apoptosis of Bronchial Epithelial Cells
- The Nedd8-activating enzyme inhibitor MLN4924 suppresses colon cancer cell growth via triggering autophagy