Korean J Physiol Pharmacol.
2018 Mar;22(2):145-153. 10.4196/kjpp.2018.22.2.145.
Toll-like receptor 2 promotes neurogenesis from the dentate gyrus after photothrombotic cerebral ischemia in mice
- Affiliations
-
- 1Dental Science Research Institute, Department of Oral Physiology, School of Dentistry, Chonnam National University, Gwangju 61186, Korea. wjkim@jnu.ac.kr, jjy@jnu.ac.kr
- 2Department of Oral and Maxillofacial Surgery, School of Dentistry, Chonnam National University, Gwangju 61186, Korea.
- KMID: 2410104
- DOI: http://doi.org/10.4196/kjpp.2018.22.2.145
Abstract
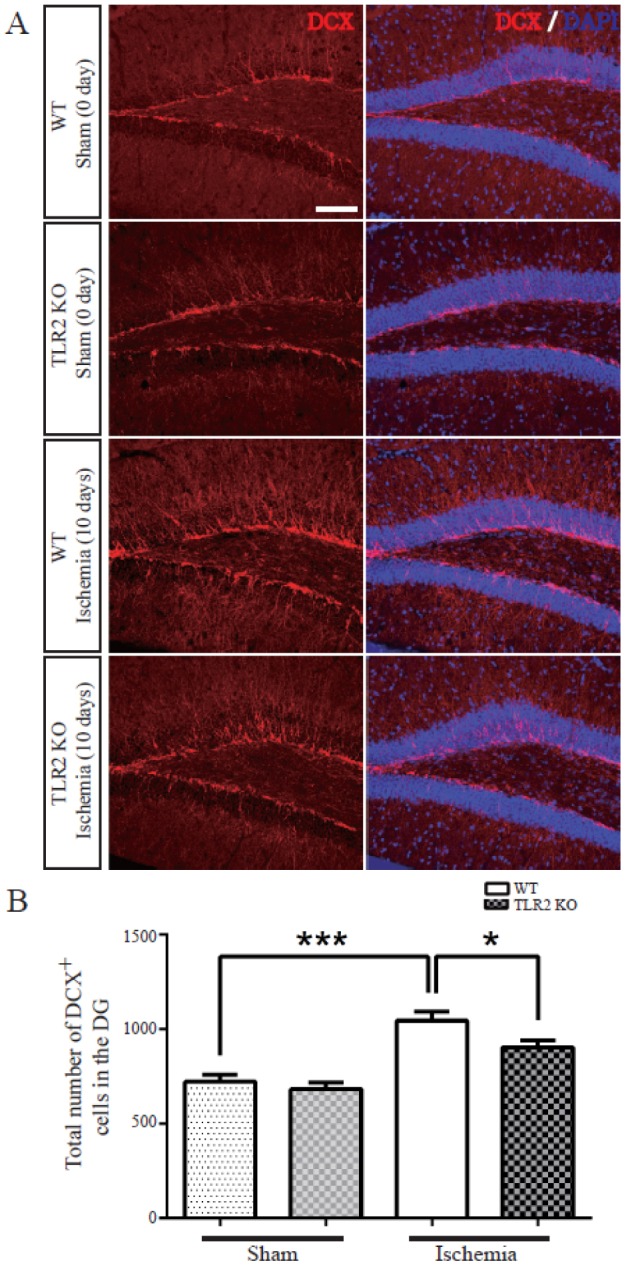
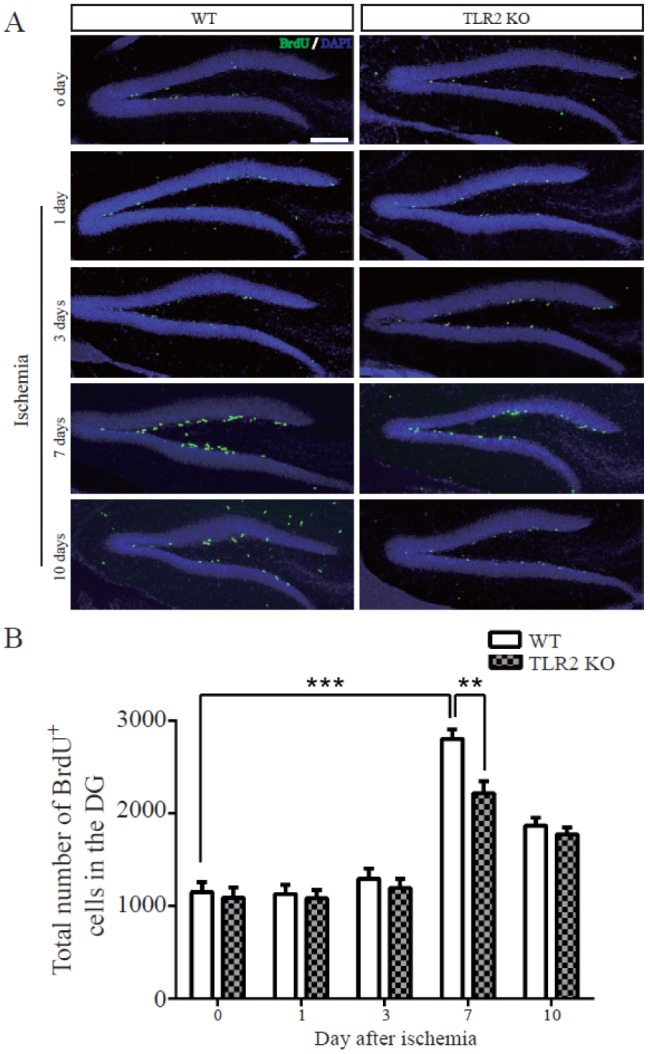
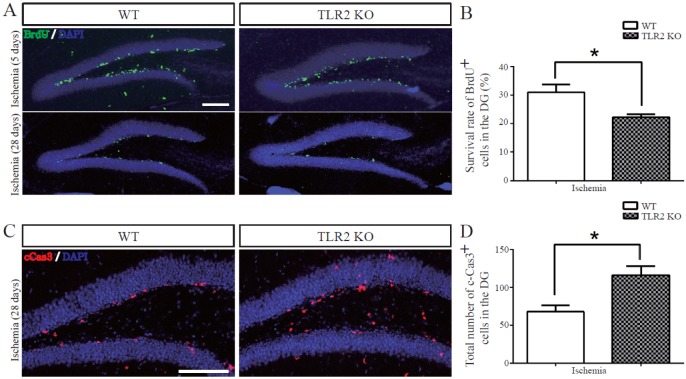
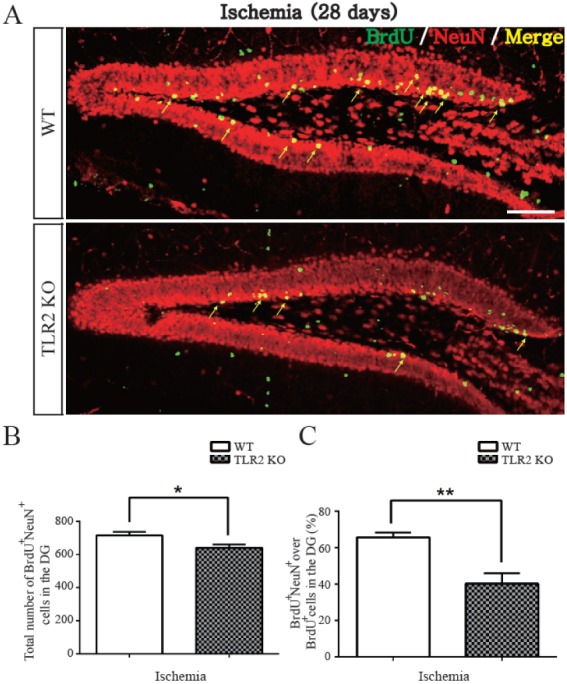
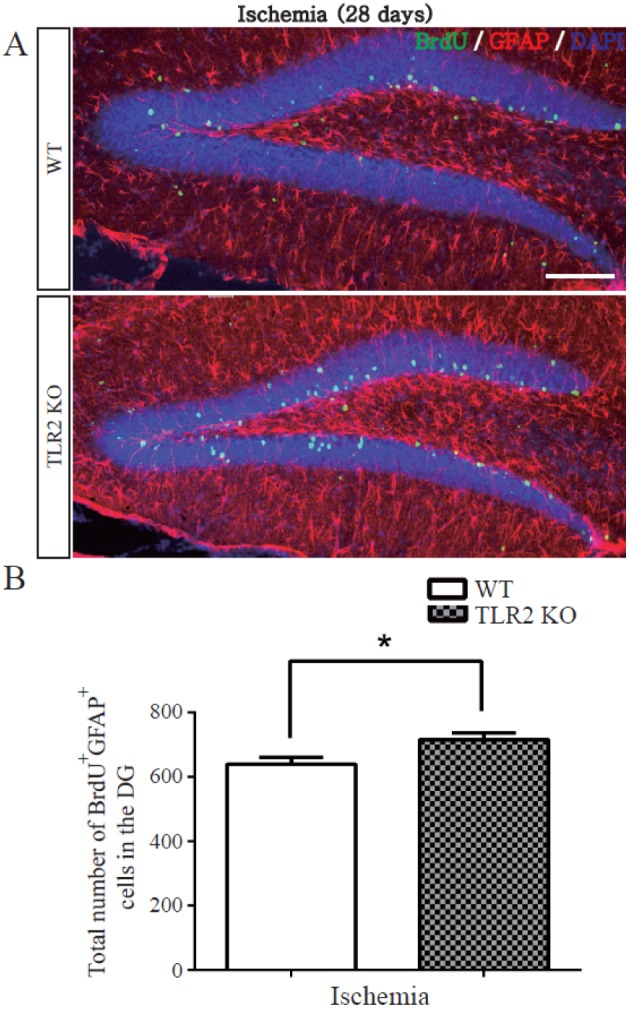
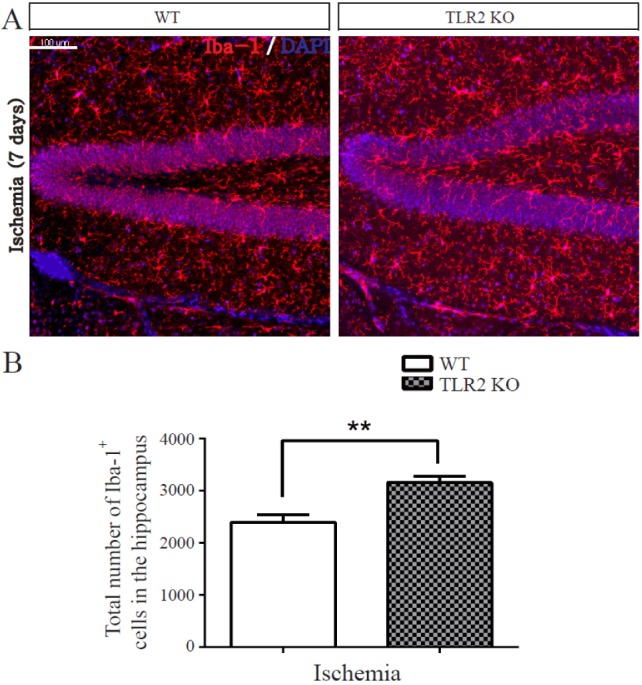
- The subgranular zone (SGZ) of hippocampal dentate gyrus (HDG) is a primary site of adult neurogenesis. Toll-like receptors (TLRs), are involved in neural system development of Drosophila and innate immune response of mammals. TLR2 is expressed abundantly in neurogenic niches such as adult mammalian hippocampus. It regulates adult hippocampal neurogenesis. However, the role of TLR2 in adult neurogenesis is not well studied in global or focal cerebral ischemia. Therefore, this study aimed to investigate the role of TLR2 in adult neurogenesis after photochemically induced cerebral ischemia. At 7 days after photothrombotic ischemic injury, the number of bromodeoxyuridine (BrdU)-positive cells was increased in both TLR2 knock-out (KO) mice and wild-type (WT) mice. However, the increment rate of BrdU-positive cells was lower in TLR2 KO mice compared to that in WT mice. The number of doublecortin (DCX) and neuronal nuclei (NeuN)-positive cells in HDG was decreased after photothrombotic ischemia in TLR2 KO mice compared to that in WT mice. The survival rate of cells in HDG was decreased in TLR2 KO mice compared to that in WT mice. In contrast, the number of cleaved-caspase 3 (apoptotic marker) and the number of GFAP (glia marker)/BrdU double-positive cells in TLR2 KO mice were higher than that in WT mice. These results suggest that TLR2 can promote adult neurogenesis from neural stem cell of hippocampal dentate gyrus through increasing proliferation, differentiation, and survival from neural stem cells after ischemic injury of the brain.
MeSH Terms
Figure
Reference
-
1. Kempermann G, Song H, Gage FH. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol. 2015; 7:a018812. PMID: 26330519.
Article2. Bond AM, Ming GL, Song H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell. 2015; 17:385–395. PMID: 26431181.
Article3. Amrein I. Adult hippocampal neurogenesis in natural populations of mammals. Cold Spring Harb Perspect Biol. 2015; 7.
Article4. Morrens J, Van Den Broeck W, Kempermann G. Glial cells in adult neurogenesis. Glia. 2012; 60:159–174. PMID: 22076934.
Article5. Drew LJ, Fusi S, Hen R. Adult neurogenesis in the mammalian hippocampus: why the dentate gyrus? Learn Mem. 2013; 20:710–729. PMID: 24255101.
Article6. Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011; 70:687–702. PMID: 21609825.
Article7. Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009; 25:253–275. PMID: 19575663.
Article8. Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005; 85:523–569. PMID: 15788705.
Article9. Garthe A, Kempermann G. An old test for new neurons: refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front Neurosci. 2013; 7:63. PMID: 23653589.
Article10. Rao MS, Hattiangady B, Shetty AK. Status epilepticus during old age is not associated with enhanced hippocampal neurogenesis. Hippocampus. 2008; 18:931–944. PMID: 18493929.
Article11. Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998; 18:7768–7778. PMID: 9742147.
Article12. Lathia JD, Okun E, Tang SC, Griffioen K, Cheng A, Mughal MR, Laryea G, Selvaraj PK, ffrench-Constant C, Magnus T, Arumugam TV, Mattson MP. Toll-like receptor 3 is a negative regulator of embryonic neural progenitor cell proliferation. J Neurosci. 2008; 28:13978–13984. PMID: 19091986.
Article13. Mouihate A. TLR4-mediated brain inflammation halts neurogenesis: impact of hormonal replacement therapy. Front Cell Neurosci. 2014; 8:146. PMID: 24904290.
Article14. Barak B, Feldman N, Okun E. Toll-like receptors as developmental tools that regulate neurogenesis during development: an update. Front Neurosci. 2014; 8:272. PMID: 25221470.
Article15. Ma Y, Haynes RL, Sidman RL, Vartanian T. TLR8: an innate immune receptor in brain, neurons and axons. Cell Cycle. 2007; 6:2859–2868. PMID: 18000403.
Article16. Matsuda T, Murao N, Katano Y, Juliandi B, Kohyama J, Akira S, Kawai T, Nakashima K. TLR9 signalling in microglia attenuates seizure-induced aberrant neurogenesis in the adult hippocampus. Nat Commun. 2015; 6:6514. PMID: 25751136.
Article17. Kaul D, Habbel P, Derkow K, Krüger C, Franzoni E, Wulczyn FG, Bereswill S, Nitsch R, Schott E, Veh R, Naumann T, Lehnardt S. Expression of Toll-like receptors in the developing brain. PLoS One. 2012; 7:e37767. PMID: 22666391.
Article18. Hemmati F, Ghasemi R, Mohamed Ibrahim N, Dargahi L, Mohamed Z, Raymond AA, Ahmadiani A. Crosstalk between insulin and Toll-like receptor signaling pathways in the central nervous system. Mol Neurobiol. 2014; 50:797–810. PMID: 24464263.
Article19. Brunn A, Mihelcic M, Carstov M, Feind L, Wieser EC, Schmidt J, Utermöhlen O, Deckert M. Toll-like receptor 2, Toll-like receptor 4, myeloid differentiation response gene 88, and Toll-IL-1 receptor domain-containing adaptor-inducing interferon-γ (TRIF) selectively regulate susceptibility of P0106-125-induced murine experimental autoimmune neuritis. Am J Pathol. 2017; 187:42–54. PMID: 27842213.
Article20. Drouin-Ouellet J, St-Amour I, Saint-Pierre M, Lamontagne-Proulx J, Kriz J, Barker RA, Cicchetti F. Toll-like receptor expression in the blood and brain of patients and a mouse model of Parkinson's disease. Int J Neuropsychopharmacol. 2014; 18.
Article21. Humann J, Mann B, Gao G, Moresco P, Ramahi J, Loh LN, Farr A, Hu Y, Durick-Eder K, Fillon SA, Smeyne RJ, Tuomanen EI. Bacterial peptidoglycan traverses the placenta to induce fetal neuroproliferation and aberrant postnatal behavior. Cell Host Microbe. 2016; 19:901.
Article22. Dzamko N, Gysbers A, Perera G, Bahar A, Shankar A, Gao J, Fu Y, Halliday GM. Toll-like receptor 2 is increased in neurons in Parkinson's disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol. 2017; 133:303–319. PMID: 27888296.
Article23. Derkow K, Krüger C, Dembny P, Lehnardt S. Microglia induce neurotoxic IL-17+ γδ T cells dependent on TLR2, TLR4, and TLR9 activation. PLoS One. 2015; 10:e0135898. PMID: 26288016.
Article24. Liu Y, Yin H, Zhao M, Lu Q. TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clin Rev Allergy Immunol. 2014; 47:136–147. PMID: 24352680.
Article25. Yonekura I, Kawahara N, Nakatomi H, Furuya K, Kirino T. A model of global cerebral ischemia in C57 BL/6 mice. J Cereb Blood Flow Metab. 2004; 24:151–158. PMID: 14747741.
Article26. Ahn JH, Shin BN, Park JH, Kim IH, Cho JH, Chen B, Lee TK, Tae HJ, Lee JC, Cho JH, Kang IJ, Kim YM, Lee YL, Won MH, Seo JY. Long-term observation of neuronal degeneration and microgliosis in the gerbil dentate gyrus after transient cerebral ischemia. J Neurol Sci. 2016; 363:21–26. PMID: 27000214.
Article27. Uemori T, Toda K, Seki T. Seizure severity-dependent selective vulnerability of the granule cell layer and aberrant neurogenesis in the rat hippocampus. Hippocampus. 2017; 27:1054–1068. PMID: 28608989.
Article28. Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. 2017; 7:278–287. PMID: 28624203.
Article29. Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007; 353:509–514. PMID: 17188246.
Article30. Ziegler G, Harhausen D, Schepers C, Hoffmann O, Röhr C, Prinz V, König J, Lehrach H, Nietfeld W, Trendelenburg G. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Commun. 2007; 359:574–579. PMID: 17548055.
Article31. Seong KJ, Lee HG, Kook MS, Ko HM, Jung JY, Kim WJ. Epigallocatechin-3-gallate rescues LPS-impaired adult hippocampal neurogenesis through suppressing the TLR4-NF-κB signaling pathway in mice. Korean J Physiol Pharmacol. 2016; 20:41–51. PMID: 26807022.
Article32. Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985; 17:497–504. PMID: 4004172.
Article33. Seong KJ, Lee HG, Kook MS, Ko HM, Jung JY, Kim WJ. Epigallocatechin-3-gallate rescues LPS-impaired adult hippocampal neurogenesis through suppressing the TLR4-NF-κB signaling pathway in mice. Korean J Physiol Pharmacol. 2016; 20:41–51. PMID: 26807022.
Article34. Gemma C, Bachstetter AD. The role of microglia in adult hippocampal neurogenesis. Front Cell Neurosci. 2013; 7:229. PMID: 24319411.
Article35. Valanne S, Wang JH, Rämet M. The drosophila toll signaling pathway. J Immunol. 2011; 186:649–656. PMID: 21209287.36. Quintin J, Asmar J, Matskevich AA, Lafarge MC, Ferrandon D. The Drosophila Toll pathway controls but does not clear Candida glabrata infections. J Immunol. 2013; 190:2818–2827. PMID: 23401590.37. Wang Y, Chen L, Tian Z, Shen X, Wang X, Wu H, Wang Y, Zou J, Liang J. CRISPR-Cas9 mediated gene knockout in human coronary artery endothelial cells reveals a pro-inflammatory role of TLR2. Cell Biol Int. 2018; 42:187–193. PMID: 28986953.
Article38. Lombardo E, DelaRosa O, Mancheño-Corvo P, Menta R, Ramírez C, Büscher D. Toll-like receptor-mediated signaling in human adiposederived stem cells: implications for immunogenicity and immunosuppressive potential. Tissue Eng Part A. 2009; 15:1579–1589. PMID: 19061425.
Article39. Hwang SH, Cho HK, Park SH, Lee W, Lee HJ, Lee DC, Oh JH, Park SH, Kim TG, Sohn HJ, Kang JM, Kim SW. Toll like receptor 3 & 4 responses of human turbinate derived mesenchymal stem cells: stimulation by double stranded RNA and lipopolysaccharide. PLoS One. 2014; 9:e101558. PMID: 25004159.40. Okun E, Griffioen KJ, Son TG, Lee JH, Roberts NJ, Mughal MR, Hutchison E, Cheng A, Arumugam TV, Lathia JD, van Praag H, Mattson MP. TLR2 activation inhibits embryonic neural progenitor cell proliferation. J Neurochem. 2010; 114:462–474. PMID: 20456021.
Article41. Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997; 17:3727–3738. PMID: 9133393.
Article42. Cameron HA, McKay R. Stem cells and neurogenesis in the adult brain. Curr Opin Neurobiol. 1998; 8:677–680. PMID: 9811628.43. Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005; 75:321–341. PMID: 15913880.
Article44. Taupin P. Neurogenesis in the adult central nervous system. C R Biol. 2006; 329:465–475. PMID: 16797452.
Article45. Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002; 110:429–441. PMID: 12202033.
Article46. Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schäbitz WR. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005; 115:2083–2098. PMID: 16007267.
Article47. Kobayashi T, Ahlenius H, Thored P, Kobayashi R, Kokaia Z, Lindvall O. Intracerebral infusion of glial cell line-derived neurotrophic factor promotes striatal neurogenesis after stroke in adult rats. Stroke. 2006; 37:2361–2367. PMID: 16873711.
Article48. Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007; 9:1081–1088. PMID: 17704767.
Article49. Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res Rev. 2009; 59:278–292. PMID: 18822314.
Article50. Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006; 24:801–812. PMID: 16782035.
Article51. Sarnico I, Lanzillotta A, Benarese M, Alghisi M, Baiguera C, Battistin L, Spano P, Pizzi M. NF-kappaB dimers in the regulation of neuronal survival. Int Rev Neurobiol. 2009; 85:351–362. PMID: 19607980.52. Chen C, Edelstein LC, Gélinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L). Mol Cell Biol. 2000; 20:2687–2695. PMID: 10733571.53. Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999; 285:736–739. PMID: 10426996.
Article54. Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem. 2001; 276:5197–5203. PMID: 11083876.55. Conti L, Lanzardo S, Arigoni M, Antonazzo R, Radaelli E, Cantarella D, Calogero RA, Cavallo F. The noninflammatory role of high mobility group box 1/Toll-like receptor 2 axis in the self-renewal of mammary cancer stem cells. FASEB J. 2013; 27:4731–4744. PMID: 23970797.
Article56. Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002; 417:39–44. PMID: 11986659.
Article57. Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006; 9:268–275. PMID: 16415867.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Change of peroxisome proliferator-activated receptor gamma expression pattern in the gerbil dentate gyrus after transient global cerebral ischemia
- The Effect of Transient Global Ischemia on the Rat Dentate Gyrus: Apoptosis in the Granular Zone and Neurogenesis in the Subgranular Zone
- Neurogenesis and Psychiatry: Focusing on Mood Disorders
- Repeated restraint stress promotes hippocampal neuronal cell ciliogenesis and proliferation in mice
- Hippocampal Neurogenesis and Phenotypic Differentiation after Pilocarpine-Induced Seizures in Young Mice