Lab Anim Res.
2018 Dec;34(4):203-210. 10.5625/lar.2018.34.4.203.
Repeated restraint stress promotes hippocampal neuronal cell ciliogenesis and proliferation in mice
- Affiliations
-
- 1College of Pharmacy, Dongguk University, Goyang, Korea.
- 2Department of Biochemistry, College of Life Science and Biotechnology, Yonsei University, Seoul, Korea. kohw@yonsei.ac.kr
- KMID: 2459296
- DOI: http://doi.org/10.5625/lar.2018.34.4.203
Abstract
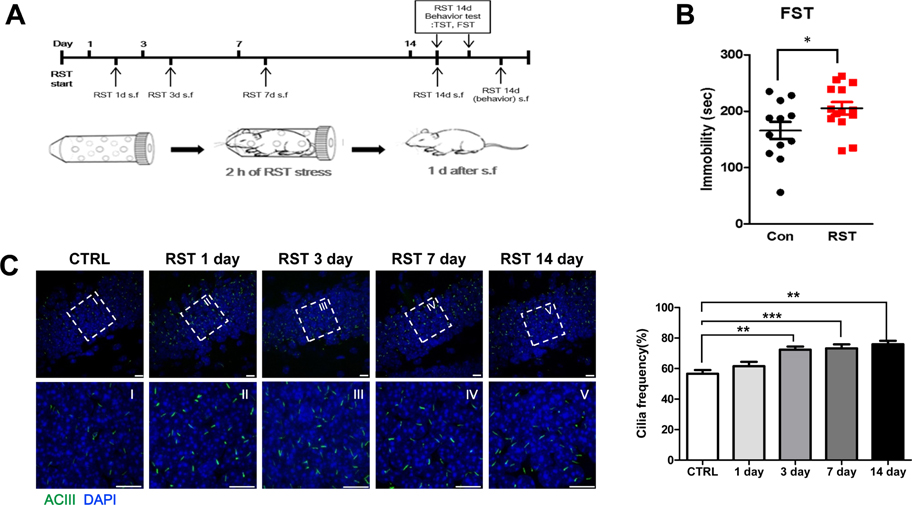
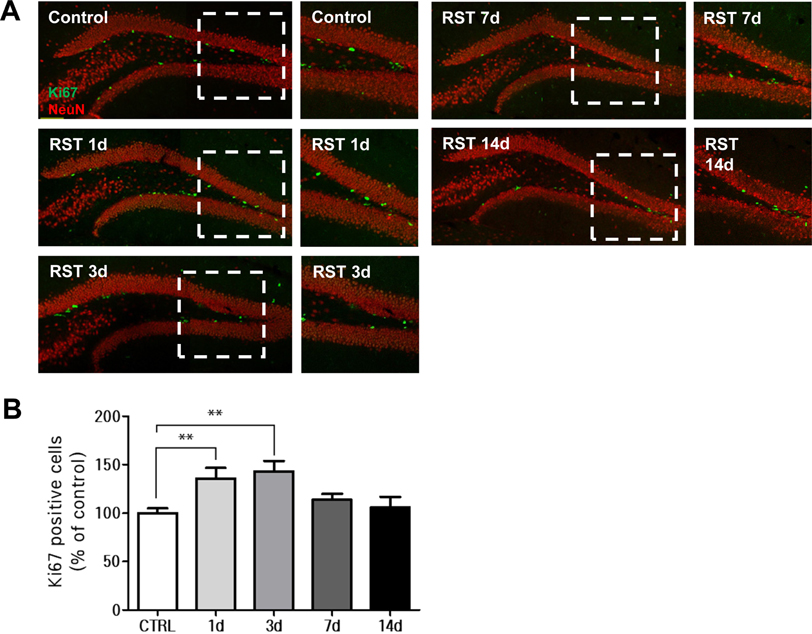
- Stress severely disturbs physiological and mental homeostasis which includes adult neurogenesis in hippocampus. Neurogenesis in hippocampus is a key feature to adapt to environmental changes and highly regulated by multiple cellular signaling pathways. The primary cilium is a cellular organelle, which acts as a signaling center during development and neurogenesis in adult mice. However, it is not clear how the primary cilia are involved in the process of restraint (RST) stress response. Using a mouse model, we examined the role of primary cilia in repeated and acute RST stress response. Interestingly, RST stress increased the number of ciliated cells in the adult hippocampal dentate gyrus (DG). In our RST model, cell proliferation in the DG also increased in a time-dependent manner. Moreover, the analysis of ciliated cells in the hippocampal DG with cell type markers indicated that cells that were ciliated in response to acute RST stress are neurons. Taken together, these findings suggest that RST stress response is closely associated with an increase in the number of ciliated neurons and leads to an increase in cell proliferation.
MeSH Terms
Figure
Reference
-
1. Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997; 21(6):801–810.
Article2. Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev. 2014; 94(4):991–1026.
Article3. Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003; 6(1):21–27.
Article4. Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002; 3(6):453–462.
Article5. Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006; 8(4):383–395.
Article6. Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007; 58:145–173.
Article7. Bartolomucci A, Leopardi R. Stress and depression: preclinical research and clinical implications. PLoS One. 2009; 4(1):e4265.
Article8. Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003; 17(4):879–886.
Article9. Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994; 61(2):203–209.
Article10. Leuner B, Glasper ER, Gould E. Sexual experience promotes adult neurogenesis in the hippocampus despite an initial elevation in stress hormones. PLoS One. 2010; 5(7):e11597.
Article11. Ko HW. The primary cilium as a multiple cellular signaling scaffold in development and disease. BMB Rep. 2012; 45(8):427–432.
Article12. Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: Recent developments. Neurosci Biobehav Rev. 2009; 33(7):1089–1098.
Article13. Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009; 15(9):1062–1065.
Article14. Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977; 229(2):327–336.15. Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 39(1):112–119.
Article16. Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006; 16(3):233–238.
Article17. Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008; 11(3):277–284.
Article18. Han YM, Kang GM, Byun K, Ko HW, Kim J, Shin MS, Kim HK, Gil SY, Yu JH, Lee B, Kim MS. Leptin-promoted cilia assembly is critical for normal energy balance. J Clin Invest. 2014; 124(5):2193–2197.
Article19. Bain MJ, Dwyer SM, Rusak B. Restraint stress affects hippocampal cell proliferation differently in rats and mice. Neurosci Lett. 2004; 368(1):7–10.
Article20. Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003; 13(5):543–550.
Article21. Seri B, García-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004; 478(4):359–378.
Article22. Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010; 11(5):331–344.
Article23. Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol. 2008; 85:225–260.24. Eichers ER, Abd-El-Barr MM, Paylor R, Lewis RA, Bi W, Lin X, Meehan TP, Stockton DW, Wu SM, Lindsay E, Justice MJ, Beales PL, Katsanis N, Lupski JR. Phenotypic characterization of Bbs4 null mice reveals age-dependent penetrance and variable expressivity. Hum Genet. 2006; 120(2):211–226.
Article25. Wang Z, Phan T, Storm DR. The type 3 adenylyl cyclase is required for novel object learning and extinction of contextual memory: role of cAMP signaling in primary cilia. J Neurosci. 2011; 31(15):5557–5561.
Article26. Veena J, Srikumar BN, Mahati K, Bhagya V, Raju TR, Shankaranarayana Rao BS. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J Neurosci Res. 2009; 87(4):831–843.
Article27. Nagata K, Nakashima-Kamimura N, Mikami T, Ohsawa I, Ohta S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology. 2009; 34(2):501–508.
Article28. Kirby ED, Muroy SE, Sun WG, Covarrubias D, Leong MJ, Barchas LA, Kaufer D. Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. Elife. 2013; 2:e00362.
Article29. Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003; 39(6):937–950.
Article30. Amador-Arjona A, Elliott J, Miller A, Ginbey A, Pazour GJ, Enikolopov G, Roberts AJ, Terskikh AV. Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory. J Neurosci. 2011; 31(27):9933–9944.
Article31. Sannino G, Pasqualini L, Ricciardelli E, Montilla P, Soverchia L, Ruggeri B, Falcinelli S, Renzi A, Ludka C, Kirchner T, Grünewald TG, Ciccocioppo R, Ubaldi M, Hardiman G. Acute stress enhances the expression of neuroprotection- and neurogenesis-associated genes in the hippocampus of a mouse restraint model. Oncotarget. 2016; 7(8):8455–8465.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of repeated restraint stress on platelet endothelial cell adhesion molecule-1 immunoreactivity and protein levels in the gerbil hippocampus after transient cerebral ischemia
- The Effects of Repeated Restraint Stress on the Synaptic Plasticity in the Inner Molecular Layer of Mouse Dentate Gyrus
- Odor Enrichment Increases Hippocampal Neuron Numbers in Mouse
- Hyperoxygenation Ameliorates Stress-induced Neuronal and Behavioral Deficits
- Effects of Diazepam on Restraint Stress-induced Fos Expression in the Rat Brain