J Korean Orthop Assoc.
2018 Apr;53(2):143-151. 10.4055/jkoa.2018.53.2.143.
The Role of Beta-Tricalcium Phosphate-Hydrogel Scaffold and Mesenchymal Stem Cells on Neogenic Bone Formation
- Affiliations
-
- 1Research Institute of Medical Science, The Catholic University of Korea, St. Vincent Hospital, Suwon, Korea.
- 2Department of Orthopedic Surgery, The Catholic University of Korea, St. Vincent Hospital, Suwon, Korea. ykang@cmcnu.or.kr
- KMID: 2410062
- DOI: http://doi.org/10.4055/jkoa.2018.53.2.143
Abstract
- PURPOSE
The purpose of this paper was to determine the ability of a mixture consisting of mesenchymal stem cells, beta-tricalcium phosphate β-TCP), and hydrogel, to support cells and form new tissue.
MATERIALS AND METHODS
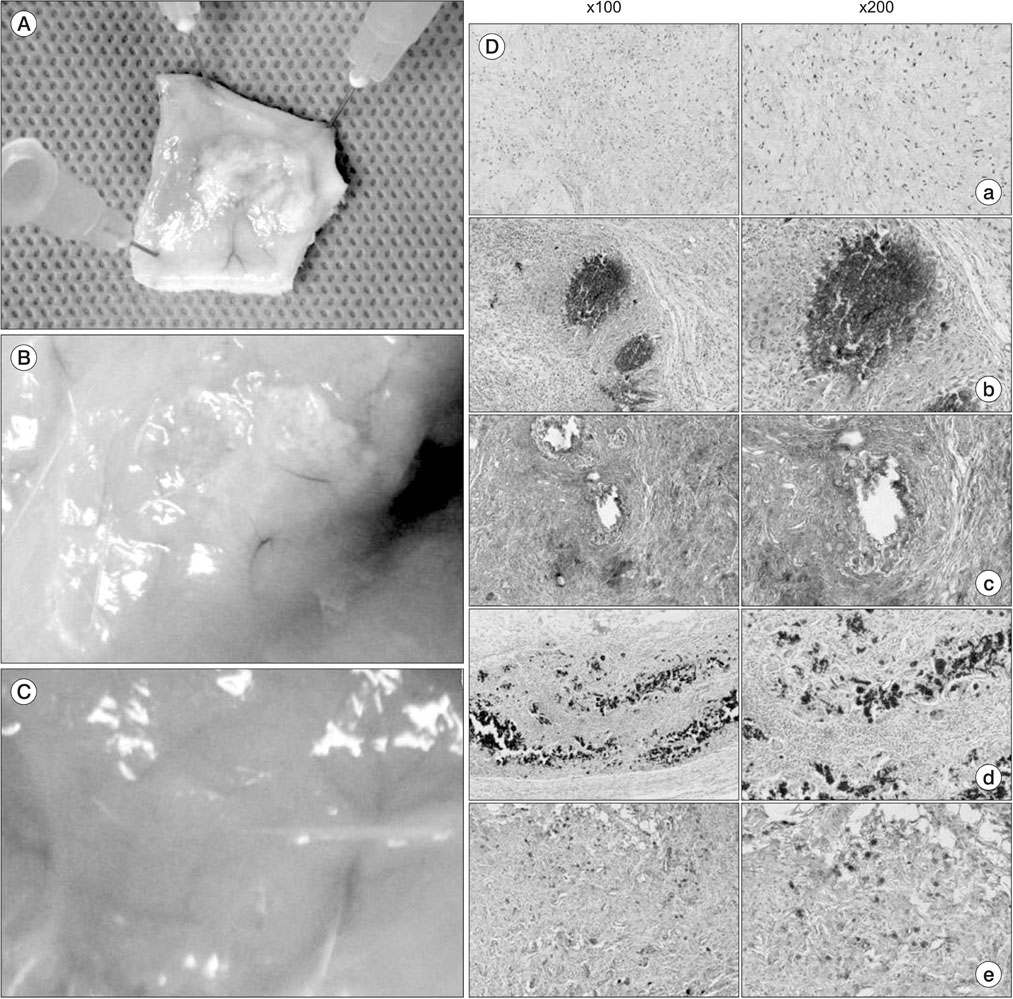
A composite was produced by adding β-TCP to hydrogel, and mesenchymal stem cells were cultivated in the composite. Then, reverse transcription polymerase chain reaction (RT-PCR) was conducted to measure the level of gene expression for the new bone formation in the cells. Moreover, a composite in which the mesenchymal stem cells were added was injected into the subcutaneous fat of sprague-dawley rats. After four weeks, H&E, Masson trichrome, silver nitrate staining, and osterix immunohistochemical staining were conducted by taking the tissue to evaluate whether the composite supported mesenchymal stem cells and formed new tissue.
RESULTS
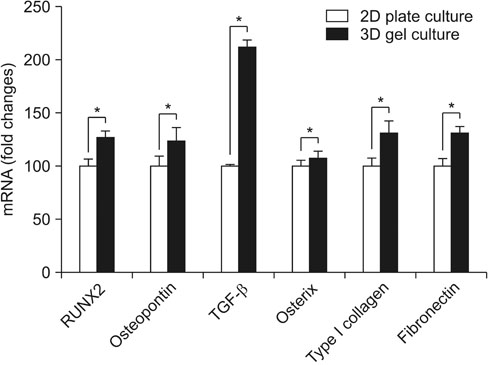
By using RT-PCR, we found that the level of gene expression became significantly higher in 3-dimensional gel culture with RUNX2 by 1.26 times, with osteopontin by 1.23 times, transforming growth factor-β by 2.12 times, osterix by 1.07 times, type I collagen by 1.3 times, and fibronectin by 1.3 times. In the animal experiment in which a composite was transplanted into the subcutaneous fat, newly formed tissue was observed. Also, it was found that the composite prevented mesenchymal stem cells from leaving and formed new tissue. Osteogenic differentiation cells in the tissue was observed through osterix immunostaining.
CONCLUSION
It was identified that the composite prevented mesenchymal stem cells dispersal and contributed to the formation of neogenic tissue. Therefore we conclude that the composite plays a role of a scaffold to support the implanted cells and form neogenic tissue more effectively.
MeSH Terms
Figure
Reference
-
1. Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005; 36:Suppl 3. S20–S27.
Article2. Cypher TJ, Grossman JP. Biological principles of bone graft healing. J Foot Ankle Surg. 1996; 35:413–417.
Article3. Carter G. Harvesting and implanting allograft bone. AORN J. 1999; 70:660–670.
Article4. Araújo M, Linder E, Lindhe J. Effect of a xenograft on early bone formation in extraction sockets: an experimental study in dog. Clin Oral Implants Res. 2009; 20:1–6.
Article5. La Gatta A, De Rosa A, Laurienzo P, Malinconico M, De Rosa M, Schiraldi C. A novel injectable poly(epsilon-caprolactone)/ calcium sulfate system for bone regeneration: synthesis and characterization. Macromol Biosci. 2005; 5:1108–1117.6. Liao HT, Chen CT. Osteogenic potential: comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells. 2014; 6:288–295.
Article7. Zipori D. Mesenchymal stem cells: harnessing cell plasticity to tissue and organ repair. Blood Cells Mol Dis. 2004; 33:211–215.
Article8. Ye X, Zhang P, Xue S, Xu Y, Tan J, Liu G. Adipose-derived stem cells alleviate osteoporosis by enhancing osteogenesis and inhibiting adipogenesis in a rabbit model. Cytotherapy. 2014; 16:1643–1655.9. Cheung HK, Han TT, Marecak DM, Watkins JF, Amsden BG, Flynn LE. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials. 2014; 35:1914–1923.
Article10. Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007; 9:204.11. Mastrogiacomo M, Muraglia A, Komlev V, et al. Tissue engineering of bone: search for a better scaffold. Orthod Craniofac Res. 2005; 8:277–284.
Article12. Ren T, Ren J, Jia X, Pan K. The bone formation in vitro and mandibular defect repair using PLGA porous scaffolds. J Biomed Mater Res A. 2005; 74:562–569.13. Park YS, Lee JY, Suh JS, et al. Selective osteogenesis by a synthetic mineral inducing peptide for the treatment of osteoporosis. Biomaterials. 2014; 35:9747–9754.
Article14. Maia FR, Barbosa M, Gomes DB, et al. Hydrogel depots for local co-delivery of osteoinductive peptides and mesenchymal stem cells. J Control Release. 2014; 189:158–168.
Article15. Nagahara T, Yoshimatsu S, Shiba H, et al. Introduction of a mixture of β-tricalcium phosphate into a complex of bone marrow mesenchymal stem cells and type I collagen can augment the volume of alveolar bone without impairing cementum regeneration. J Periodontol. 2015; 86:456–464.
Article16. Murphy KC, Hughbanks ML, Binder BY, Vissers CB, Leach JK. Engineered fibrin gels for parallel stimulation of mesenchymal stem cell proangiogenic and osteogenic potential. Ann Biomed Eng. 2015; 43:2010–2021.
Article17. He X, Liu Y, Yuan X, Lu L. Enhanced healing of rat calvarial defects with MSCs loaded on BMP-2 releasing chitosan/alginate/ hydroxyapatite scaffolds. PLoS One. 2014; 9:e104061.18. Cuadros TR, Erices AA, Aguilera JM. Porous matrix of calcium alginate/gelatin with enhanced properties as scaffold for cell culture. J Mech Behav Biomed Mater. 2015; 46:331–342.
Article19. Peter SJ, Miller ST, Zhu G, Yasko AW, Mikos AG. In vivo degradation of a poly(propylene fumarate)/beta-tricalcium phosphate injectable composite scaffold. J Biomed Mater Res. 1998; 41:1–7.20. Peters F, Reif D. Functional materials for bone regeneration from beta-tricalcium phosphate. Mater Sci Engine Technol. 2004; 35:203–207.
Article21. Maia FR, Fonseca KB, Rodrigues G, Granja PL, Barrias CC. Matrix-driven formation of mesenchymal stem cell-extracellular matrix microtissues on soft alginate hydrogels. Acta Biomater. 2014; 10:3197–3208.
Article22. Salama R, Weissman SL. The clinical use of combined xenografts of bone and autologous red marrow. A preliminary report. J Bone Joint Surg Br. 1978; 60:111–115.
Article23. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.
Article24. Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008; 45:115–120.
Article25. Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011; 9:12.
Article26. Han S, Zhao Y, Xiao Z, et al. The three-dimensional collagen scaffold improves the stemness of rat bone marrow mesenchymal stem cells. J Genet Genomics. 2012; 39:633–641.
Article27. Cheng L, Shi Y, Ye F, Bu H. Osteoinduction of calcium phosphate biomaterials in small animals. Mater Sci Eng C Mater Biol Appl. 2013; 33:1254–1260.
Article28. Amosi N, Zarzhitsky S, Gilsohn E, et al. Acidic peptide hydrogel scaffolds enhance calcium phosphate mineral turnover into bone tissue. Acta Biomater. 2012; 8:2466–2475.
Article29. Rustad KC, Wong VW, Sorkin M, et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials. 2012; 33:80–90.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of serum-derived albumin scaffold and canine adipose tissue-derived mesenchymal stem cells on osteogenesis in canine segmental bone defect model
- Periosteum-Derived Mesenchymal Stem Cells and Scaffolds Transplanted into Long-Bone Defects of Rabbit
- Effects of Polycaprolactone-Tricalcium Phosphate, Recombinant Human Bone Morphogenetic Protein-2 and Dog Mesenchymal Stem Cells on Bone Formation: Pilot Study in Dogs
- The Effect of beta-Tricalcium Phosphate and Deproteinized Bovine Bone on Bone Formation in the Defects of Rat Calvaria
- Effects of three-dimensionally printed polycaprolactone/β-tricalcium phosphate scaffold on osteogenic differentiation of adipose tissue- and bone marrow-derived stem cells