Korean J Physiol Pharmacol.
2018 Jan;22(1):63-70. 10.4196/kjpp.2018.22.1.63.
Cilostazol attenuates kainic acid-induced hippocampal cell death
- Affiliations
-
- 1Department of Neurosurgery, Institute of Health Sciences, Gyeongsang National University Changwon Hospital, Changwon 51472, Korea.
- 2Department of Anatomy and Convergence Medical Science, Institute of Health Sciences, Gyeongsang National University College of Medicine, Jinju 52727, Korea. anaroh@gnu.ac.kr
- 3Department of Neurosurgery, Institute of Health Sciences, Gyeongsang National University Hospital, Gyeongsang National University College of Medicine, Jinju 52727, Korea. gnuhpis@gnu.ac.kr
- KMID: 2398556
- DOI: http://doi.org/10.4196/kjpp.2018.22.1.63
Abstract
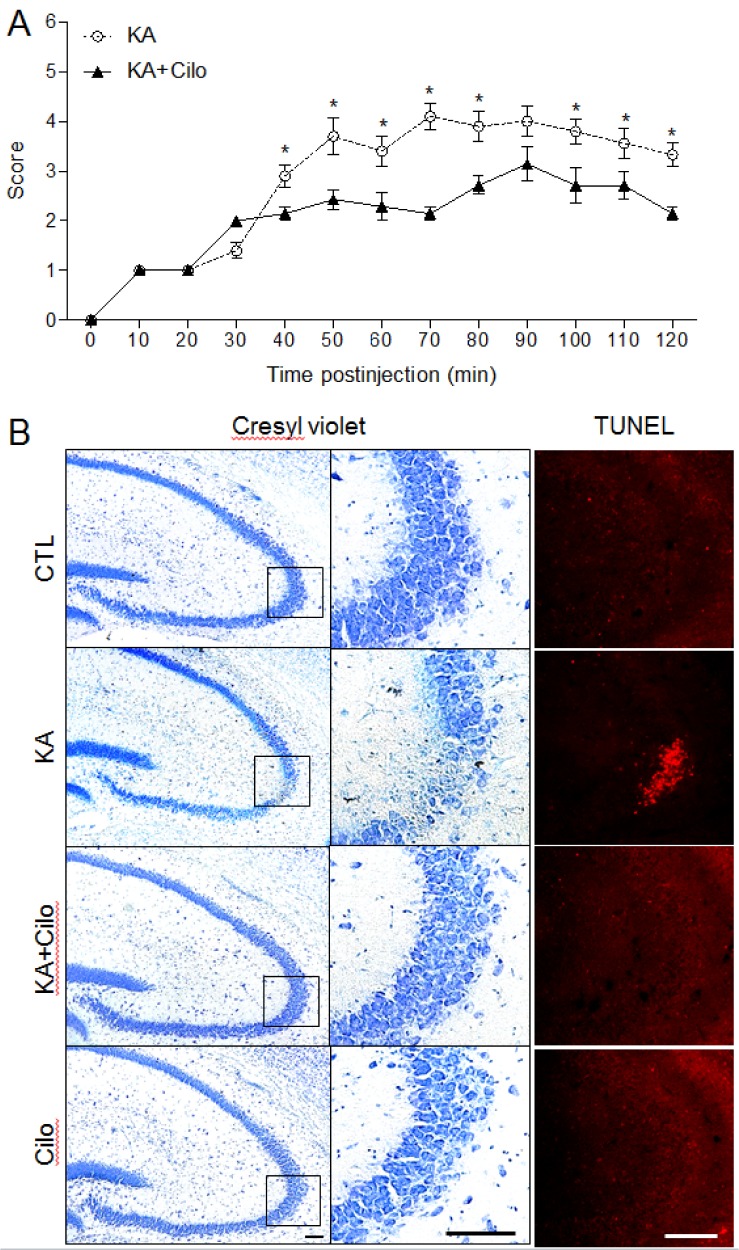
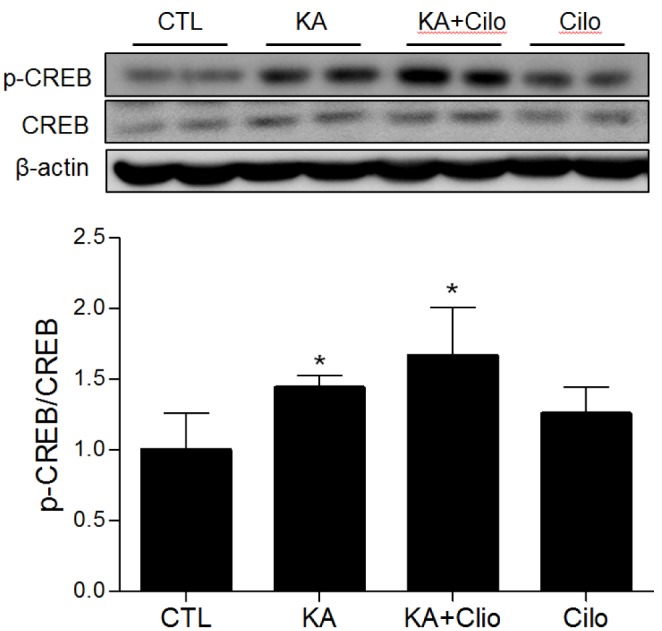
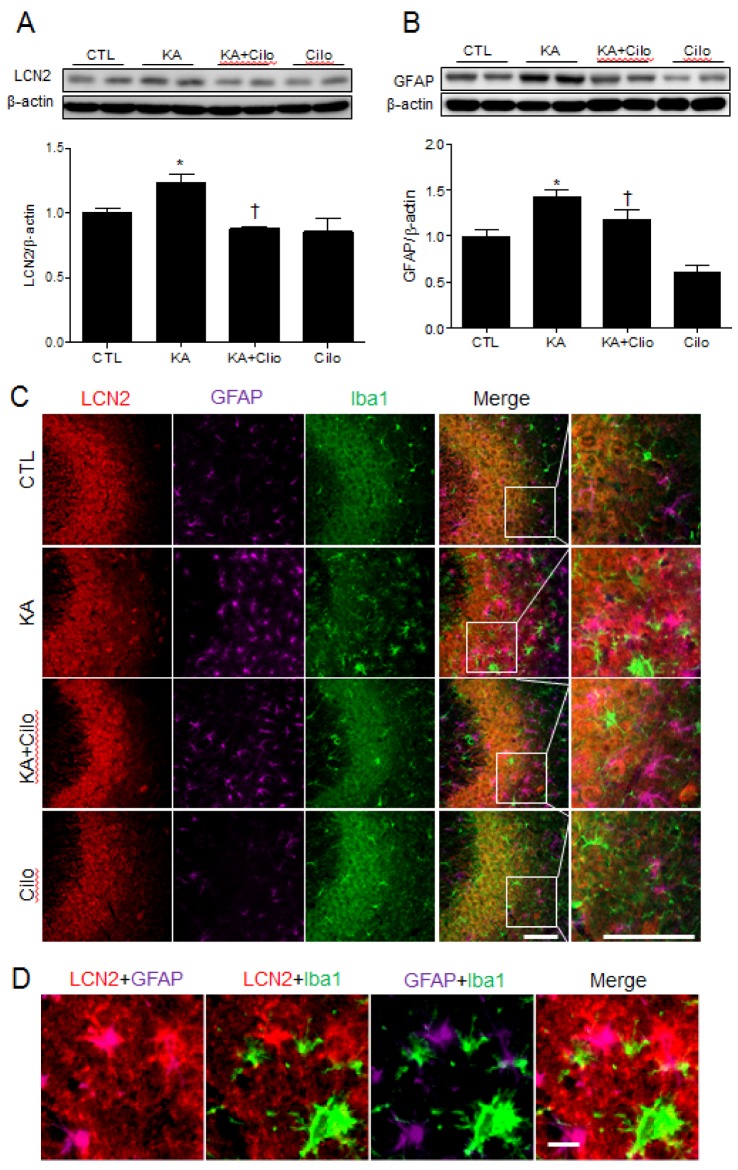
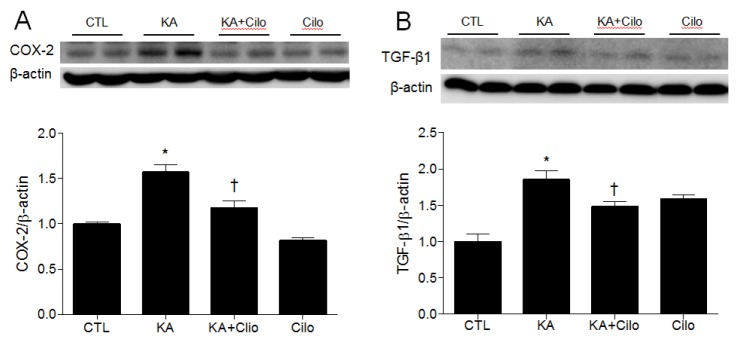
- Cilostazol is a selective inhibitor of type 3 phosphodiesterase (PDE3) and has been widely used as an antiplatelet agent. Cilostazol mediates this activity through effects on the cyclic adenosine monophosphate (cAMP) signaling cascade. Recently, it has attracted attention as a neuroprotective agent. However, little is known about cilostazol's effect on excitotoxicity induced neuronal cell death. Therefore, this study evaluated the neuroprotective effect of cilostazol treatment against hippocampal neuronal damage in a mouse model of kainic acid (KA)-induced neuronal loss. Cilostazol pretreatment reduced KA-induced seizure scores and hippocampal neuron death. In addition, cilostazol pretreatment increased cAMP response element-binding protein (CREB) phosphorylation and decreased neuroinflammation. These observations suggest that cilostazol may have beneficial therapeutic effects on seizure activity and other neurological diseases associated with excitotoxicity.
Keyword
MeSH Terms
Figure
Reference
-
1. Kimura Y, Tani T, Kanbe T, Watanabe K. Effect of cilostazol on platelet aggregation and experimental thrombosis. Arzneimittelforschung. 1985; 35:1144–1149. PMID: 4074426.2. Kambayashi J, Liu Y, Sun B, Shakur Y, Yoshitake M, Czerwiec F. Cilostazol as a unique antithrombotic agent. Curr Pharm Des. 2003; 9:2289–2302. PMID: 14529391.
Article3. Miyamoto N, Tanaka R, Shimura H, Watanabe T, Mori H, Onodera M, Mochizuki H, Hattori N, Urabe T. Phosphodiesterase III inhibition promotes differentiation and survival of oligodendrocyte progenitors and enhances regeneration of ischemic white matter lesions in the adult mammalian brain. J Cereb Blood Flow Metab. 2010; 30:299–310. PMID: 19826432.
Article4. Lee JH, Park SY, Shin YW, Hong KW, Kim CD, Sung SM, Kim KY, Lee WS. Neuroprotection by cilostazol, a phosphodiesterase type 3 inhibitor, against apoptotic white matter changes in rat after chronic cerebral hypoperfusion. Brain Res. 2006; 1082:182–191. PMID: 16516167.
Article5. Qi DS, Tao JH, Zhang LQ, Li M, Wang M, Qu R, Zhang SC, Liu P, Liu F, Miu JC, Ma JY, Mei XY, Zhang F. Neuroprotection of Cilostazol against ischemia/reperfusion-induced cognitive deficits through inhibiting JNK3/caspase-3 by enhancing Akt1. Brain Res. 2016; 1653:67–74. PMID: 27769787.
Article6. Nadler JV, Perry BW, Cotman CW. Intraventricular kainic acid preferentially destroys hippocampal pyramidal cells. Nature. 1978; 271:676–677. PMID: 625338.
Article7. Rizzi M, Perego C, Aliprandi M, Richichi C, Ravizza T, Colella D, Velískŏvá J, Moshe SL, De Simoni MG, Vezzani A. Glia activation and cytokine increase in rat hippocampus by kainic acid-induced status epilepticus during postnatal development. Neurobiol Dis. 2003; 14:494–503. PMID: 14678765.
Article8. Kim H, Lee JY, Park KJ, Kim WH, Roh GS. A mitochondrial division inhibitor, Mdivi-1, inhibits mitochondrial fragmentation and attenuates kainic acid-induced hippocampal cell death. BMC Neurosci. 2016; 17:33. PMID: 27287829.
Article9. Shin HJ, Lee JY, Son E, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Curcumin attenuates the kainic acid-induced hippocampal cell death in the mice. Neurosci Lett. 2007; 416:49–54. PMID: 17300872.
Article10. Chia WJ, Dawe GS, Ong WY. Expression and localization of the iron-siderophore binding protein lipocalin 2 in the normal rat brain and after kainate-induced excitotoxicity. Neurochem Int. 2011; 59:591–599. PMID: 21683107.
Article11. Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010; 7:354–365. PMID: 20880500.
Article12. Lee S, Park JY, Lee WH, Kim H, Park HC, Mori K, Suk K. Lipocalin-2 is an autocrine mediator of reactive astrocytosis. J Neurosci. 2009; 29:234–249. PMID: 19129400.
Article13. Borkham-Kamphorst E, Drews F, Weiskirchen R. Induction of lipocalin-2 expression in acute and chronic experimental liver injury moderated by pro-inflammatory cytokines interleukin-1β through nuclear factor-κB activation. Liver Int. 2011; 31:656–665. PMID: 21457438.
Article14. Yoneyama M, Tanaka M, Hasebe S, Yamaguchi T, Shiba T, Ogita K. Beneficial effect of cilostazol-mediated neuronal repair following trimethyltin-induced neuronal loss in the dentate gyrus. J Neurosci Res. 2015; 93:56–66. PMID: 25139675.
Article15. Jeong EA, Jeon BT, Shin HJ, Kim N, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Ketogenic diet-induced peroxisome proliferator-activated receptor-γ activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp Neurol. 2011; 232:195–202. PMID: 21939657.
Article16. Kim YR, Kim HN, Hong KW, Shin HK, Choi BT. Anti-depressant effects of phosphodiesterase 3 inhibitor cilostazol in chronic mild stress-treated mice after ischemic stroke. Psychopharmacology (Berl). 2016; 233:1055–1066. PMID: 26686236.
Article17. Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985; 14:375–403. PMID: 2859548.
Article18. Morgan TE, Nichols NR, Pasinetti GM, Finch CE. TGF-β1 mRNA increases in macrophage/microglial cells of the hippocampus in response to deafferentation and kainic acid-induced neurodegeneration. Exp Neurol. 1993; 120:291–301. PMID: 8491285.
Article19. Tanaka Y, Tanaka R, Liu M, Hattori N, Urabe T. Cilostazol attenuates ischemic brain injury and enhances neurogenesis in the subventricular zone of adult mice after transient focal cerebral ischemia. Neuroscience. 2010; 171:1367–1376. PMID: 20933581.
Article20. Liu Y, Shakur Y, Yoshitake M, Kambayashi Ji J. Cilostazol (pletal): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc Drug Rev. 2001; 19:369–386. PMID: 11830753.
Article21. Watanabe T, Zhang N, Liu M, Tanaka R, Mizuno Y, Urabe T. Cilostazol protects against brain white matter damage and cognitive impairment in a rat model of chronic cerebral hypoperfusion. Stroke. 2006; 37:1539–1545. PMID: 16645134.
Article22. Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002; 22:3673–3682. PMID: 11978843.
Article23. Tanis KQ, Duman RS, Newton SS. CREB binding and activity in brain: regional specificity and induction by electroconvulsive seizure. Biol Psychiatry. 2008; 63:710–720. PMID: 17936724.
Article24. Moore AN, Waxham MN, Dash PK. Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. J Biol Chem. 1996; 271:14214–14220. PMID: 8662977.
Article25. Pardo L, Schlüter A, Valor LM, Barco A, Giralt M, Golbano A, Hidalgo J, Jia P, Zhao Z, Jové M, Portero-Otin M, Ruiz M, Giménez-Llort L, Masgrau R, Pujol A, Galea E. Targeted activation of CREB in reactive astrocytes is neuroprotective in focal acute cortical injury. Glia. 2016; 64:853–874. PMID: 26880229.
Article26. Watanabe T, Zhang N, Liu M, Tanaka R, Mizuno Y, Urabe T. Cilostazol protects against brain white matter damage and cognitive impairment in a rat model of chronic cerebral hypoperfusion. Stroke. 2006; 37:1539–1545. PMID: 16645134.
Article27. Tanaka K, Nogawa S, Ito D, Suzuki S, Dembo T, Kosakai A, Fukuuchi Y. Activated phosphorylation of cyclic AMP response element binding protein is associated with preservation of striatal neurons after focal cerebral ischemia in the rat. Neuroscience. 2000; 100:345–354. PMID: 11008172.
Article28. Chen Z, Duan RS, Quezada HC, Mix E, Nennesmo I, Adem A, Winblad B, Zhu J. Increased microglial activation and astrogliosis after intranasal administration of kainic acid in C57BL/6 mice. J Neurobiol. 2005; 62:207–218. PMID: 15459893.
Article29. Stienen MN, Haghikia A, Dambach H, Thone J, Wiemann M, Gold R, Chan A, Dermietzel R, Faustmann PM, Hinkerohe D, Prochnow N. Anti-inflammatory effects of the anticonvulsant drug levetiracetam on electrophysiological properties of astroglia are mediated via TGFβ1 regulation. Br J Pharmacol. 2011; 162:491–507. PMID: 20955362.
Article30. Laping NJ, Nichols NR, Day JR, Finch CE. Corticosterone differentially regulates the bilateral response of astrocyte mRNAs in the hippocampus to entorhinal cortex lesions in male rats. Brain Res Mol Brain Res. 1991; 10:291–297. PMID: 1717807.
Article31. Prehn JH, Miller RJ. Opposite effects of TGF-β1 on rapidly- and slowly-triggered excitotoxic injury. Neuropharmacology. 1996; 35:249–256. PMID: 8783198.
Article32. Wang XS, Ong WY, Connor JR. Increase in ferric and ferrous iron in the rat hippocampus with time after kainate-induced excitotoxic injury. Exp Brain Res. 2002; 143:137–148. PMID: 11880890.
Article33. Huang E, Ong WY. Distribution of ferritin in the rat hippocampus after kainate-induced neuronal injury. Exp Brain Res. 2005; 161:502–511. PMID: 15747160.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Atorvastatin pretreatment attenuates kainic acid-induced hippocampal neuronal death via regulation of lipocalin-2-associated neuroinflammation
- Curcumin Attenuates Glial Cell Activation But Cannot Suppress Hippocampal CA3 Neuronal Cell Death in i.c.v. Kanic Acid Injection Model
- Role of gamma-aminobutyric acid B (GABA B) receptors in the regulation of kainic acid-induced cell death in mouse hippocampus
- Intracerebroventricular Kainic Acid-Induced Damage Affects Blood Glucose Level in d-glucose-fed Mouse Model
- Effect of Valproic Acid on Bcl-2, Bim, and Caspase-3 Expression in Rat Hippocampus after Kainic Acid-Induced Seizures