J Korean Ophthalmol Soc.
2019 Dec;60(12):1176-1184. 10.3341/jkos.2019.60.12.1176.
Comparison of the Toxicity of Olopatadine Anti-allergic Ophthalmic Agents on Rabbit Conjunctival Cells
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University College of Medicine, Busan, Korea. jongsool@pusan.ac.kr
- 2BioMedical Research Institute of Pusan National University Hospital, Busan, Korea.
- KMID: 2466168
- DOI: http://doi.org/10.3341/jkos.2019.60.12.1176
Abstract
- PURPOSE
To compare the in vitro toxicity of commercial olopatadine anti-allergic ophthalmic agents on cultured rabbit's conjunctival cells according to concentrations and exposure times.
METHODS
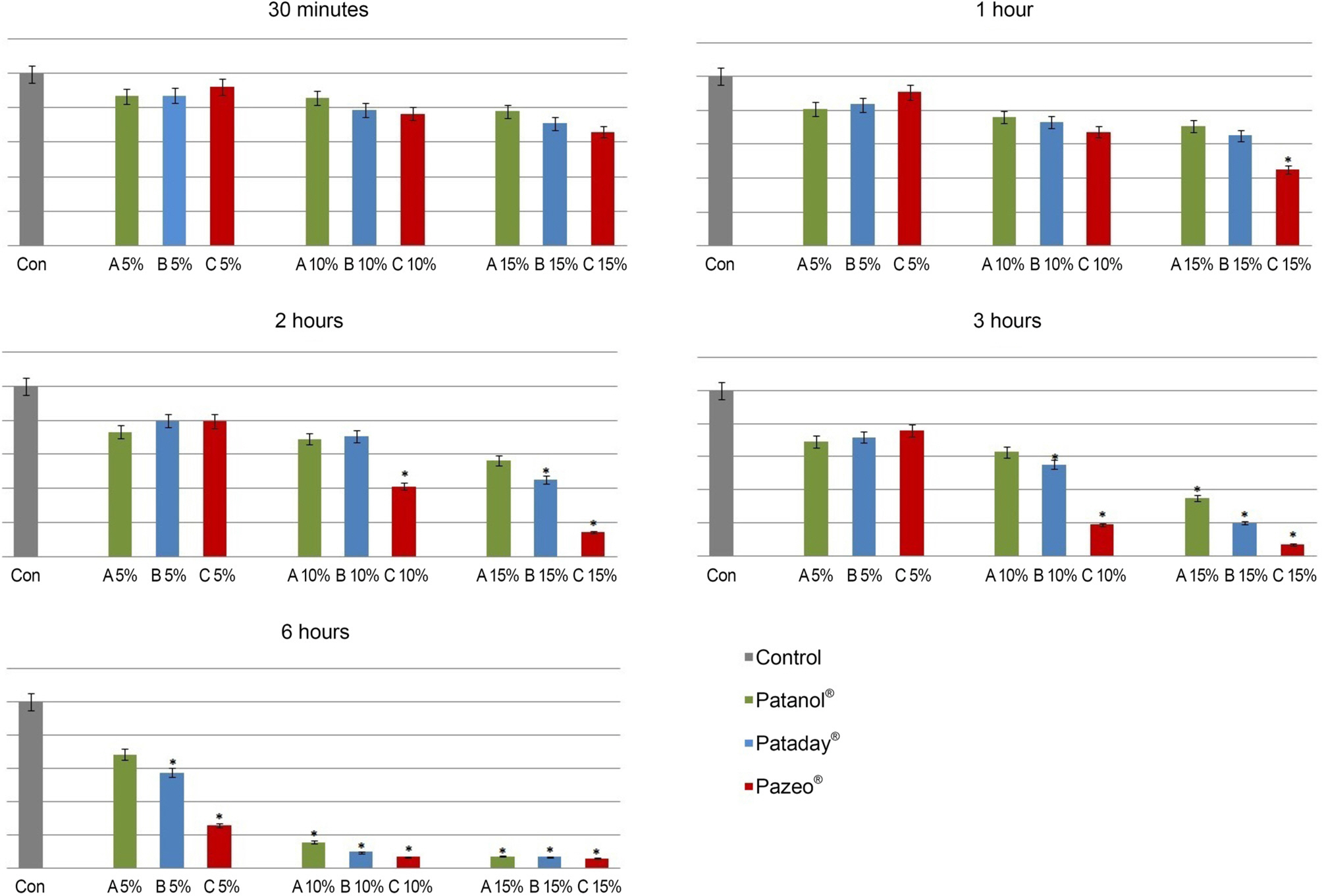
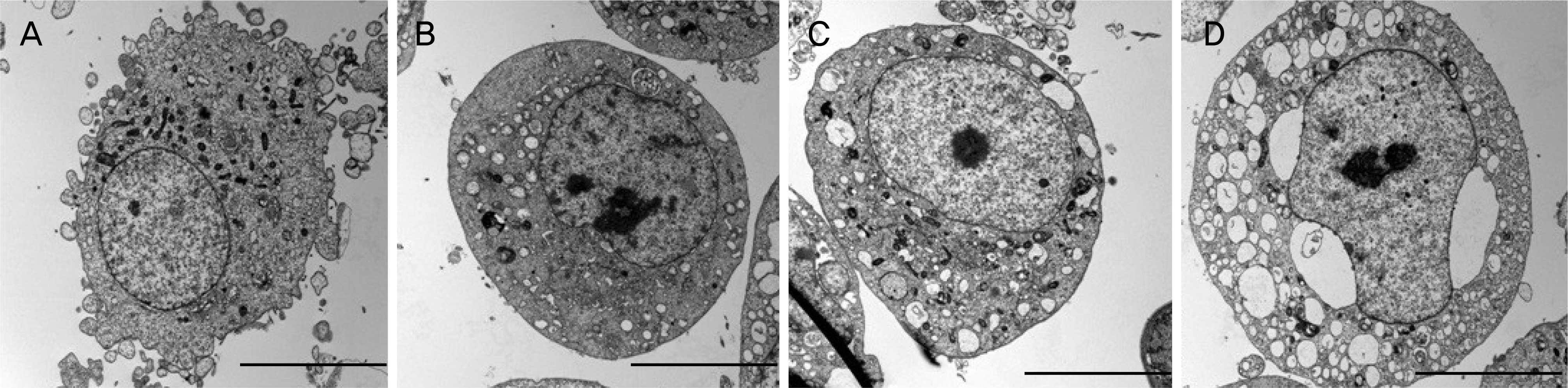
Rabbit conjunctival cells were exposed to anti-allergic olopatadine ophthalmic agents, (Patanol® [0.1% olopatadine hydrochloride; Alcon, Fort Worth, TX, USA], Pataday® [0.2% olopatadine hydrochloride; Alcon], and Pazeo® [0.7% olopatadine hydrochloride; Alcon]) at concentrations of 5%, 10%, and 15% for periods of 30 minutes, 1, 2, 3, and 6 hours, respectively. Cell proliferation and injury assays were performed using the methylthiazoltetrazolium and lactate dehydrogenase (LDH) leakage assays. We checked the composition of the three anti-allergic agents, and performed light and transmission electron microscopy to compare the morphological changes in cells.
RESULTS
The conjunctival cell proliferation was inhibited after 1 hour exposure to each olopatadine ophthalmic agent, with significant cell proliferation inhibited using 15% of each drug. The proliferation of conjunctival cells was inhibited during 6 hours of drug exposure at all concentrations of Pataday® and Pazeo®. The titer of LDH increased from 3 hours after drug exposure, but 15% Pazeo® significantly increased the LDH titer at 2 hours after drug exposure. As the concentration of the drug increased, the LDH titer also significantly increased. The cellular morphological changes of conjunctival cells were in the increasing order of Pazeo®, Pataday®, and Patanol® with a high concentration of olopatadine hydrochloride.
CONCLUSIONS
Among the anti-allergic olopatidine ophthalmic agents, higher olopatadine concentrations in the increasing order of Pazeo®, Pataday®, and Patanol® resulted in cytoplasmic damage of conjunctival cells, but there was no severe damage to the cytoplasmic or the nuclear membranes.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Bielory L. Allergic and immunologic disorders of the eye. Part I: immunology of the eye. J Allergy Clin Immunol. 2000; 106:805–16.
Article2. Bielory L. Therapeutic targets in allergic eye disease. Allergy Asthma Proc. 2001; 22:25–8.
Article3. Kidd M, McKenzie S, Steven I, et al. Efficacy and safety of abdominal eye drops in the treatment of seasonal allergic conjunctivitis. Br J Ophthalmol. 2003; 87:1206–11.4. Abelson MB, Ferzola NJ, McWhirter CL, Crampton HJ. Efficacy and safety of single‐ and multiple‐ dose ketotifen fumarate 0.025% ophthalmic solution in a pediatric population. Pediatr Allergy Immunol. 2004; 15:551–7.5. Kempuraj D, Asadi S, Zhang B, et al. Mercury induces inflammatory mediator release from human mast cells. J Neuroinflammation. 2010; 7:20.
Article6. Yamaguchi M, Azuma H, Fujihara M, et al. Generation of a considerable number of functional mast cells with a high basal level of Fcɛ RI expression from cord blood CD34+ cells by co‐ culturing them with bone marrow stromal cell line under serum‐ free conditions. Scand J Immunol. 2007; 65:581–8.7. Yanni JM, Miller ST, Gamache DA, et al. Comparative effects of topical ocular anti-allergy drugs on human conjunctival mast cells. Ann Allergy Asthma Immunol. 1997; 79:541–5.
Article8. Byon IS, Park JS, Lee JE, Lee JS. The effects of abdominal ophthalmic agents on the cultured rabbit conjunctival cells. J Korean Ophthalmol Soc. 2006; 47:1340–8.9. Mosmann T. Rapid colorimetric assay for cellular growth and abdominal: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.10. Kim SJ, Yoon TJ, Lee JE, Lee JS. Comparison of the efficacy of topical antihistamine/mast cell stabilizers in vitro. J Korean Ophthalmol Soc. 2010; 51:406–17.
Article11. Wee WR, Wang XW, McDonnell PJ. Effect of artificial tears on cultured keratocytes in vitro. Cornea. 1995; 14:273–9.
Article12. Park Y. Physiology of body fluid. Kang DH, editor. Physiology. 5th ed.Seoul: Seoul;2000. chap. 5.13. Kim K. Acid-base balance. Sung HK, KIM KH, editors. Physiology. 5th ed.Seoul: Seoul;1996. p. 326–7.14. Freshney R. The culture environment: substrate, gas phase, medium and temperature. Freshney R, editor. Culture of animal cells: a manual of basic technique. 3rd ed.New York: New York;1994. p. 71.15. Burstein NL, Klyce SD. Electrophysiologic and morphologic abdominals of ophthalmic preparations on rabbit cornea epithelium. Invest Ophthalmol Vis Sci. 1977; 16:899–911.16. Pfister RR, Burstein N. The effects of ophthalmic drugs, vehicles, and preservatives on corneal epithelium: a scanning electron microscope study. Invest Ophthalmol. 1976; 15:246–59.17. Lemp MA, Zimmerman LE. Toxic endothelial degeneration in abdominal surface disease treated with topical medications containing benzalkonium chloride. Am J Ophthalmol. 1988; 105:670–3.18. Green K, Tonjum A. Influence of various agents on corneal permeability. Am J Ophthalmol. 1971; 72:897–905.
Article19. Lee JS, Oum BS, Kim CD. Cytotoxicity of benzalkonium chloride on the corneal epithelial cell of rabbit. J Korean Ophthalmol Soc. 1998; 39:1326–33.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Olopatadine ophthalmic solution suppresses substance P release in the conjunctivitis models
- The Effects of Anti-allergic Ophthalmic Agents on the Cultured Rabbit Conjunctival Cells
- Evaluation of the Stabilization of Human Umbilical Cord Blood-Derived Mast Cells in Accordance with Ketotifen and Olopatadine Concentration
- Comparison of the Efficacy of Topical Antihistamine/Mast Cell Stabilizers in vitro
- A Case of Allergic Contact Dermatitis to Ophthalmic Ointment