Ann Dermatol.
2017 Aug;29(4):391-399. 10.5021/ad.2017.29.4.391.
Suppressive Effects of Mesenchymal Stem Cells in Adipose Tissue on Allergic Contact Dermatitis
- Affiliations
-
- 1Department of Dermatology, The Jikei University School of Medicine, Tokyo, Japan. kikutyini@jikei.ac.jp
- 2Department of Regenerative Medicine, Research Institute, National Center for Global Health and Medicine, Tokyo, Japan.
- 3Department of Dermatology, Nippon Medical School, Tokyo, Japan.
- 4Department of Dermatology, Teikyo University School of Medicine, Tokyo, Japan.
- KMID: 2388935
- DOI: http://doi.org/10.5021/ad.2017.29.4.391
Abstract
- BACKGROUND
Allergic contact dermatitis (ACD), which is accelerated by interferon (IFN)-γ and suppressed by interleukin (IL)-10 as regulators, is generally self-limited after removal of the contact allergen. Adipose tissue-derived multipotent mesenchymal stem cells (ASCs) potentially exert immunomodulatory effects. Considering that subcutaneous adipose tissue is located close to the site of ACD and includes mesenchymal stem cells (MSCs), the MSCs in adipose tissue could contribute to the self-limiting course of ACD.
OBJECTIVE
The aims of the present study were to elucidate the effects of MSCs in adipose tissue on ACD and to examine any cytokine-mediated mechanisms involved.
METHODS
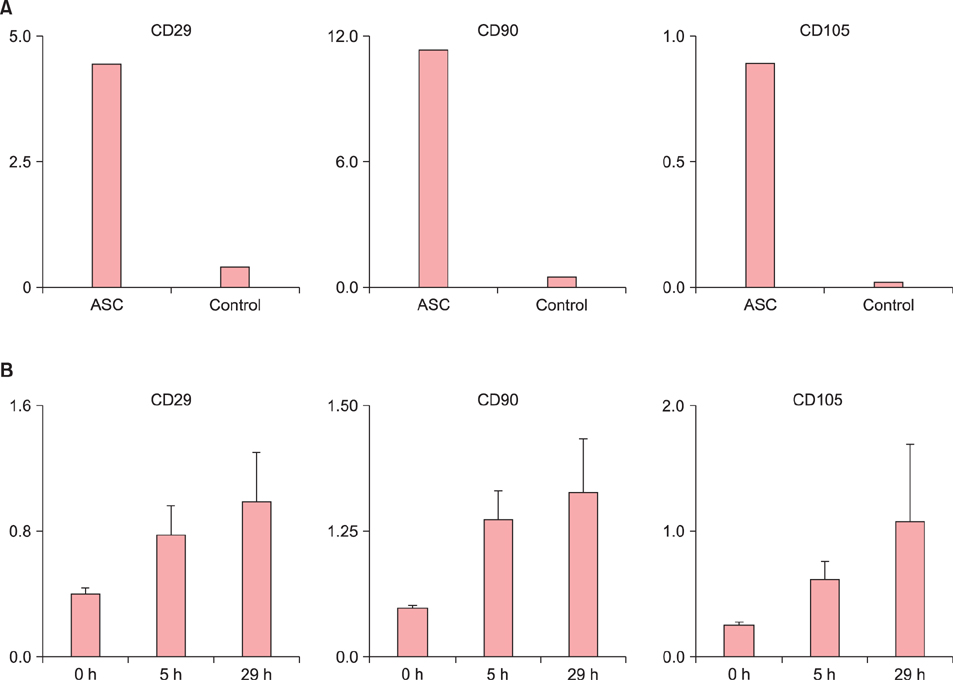
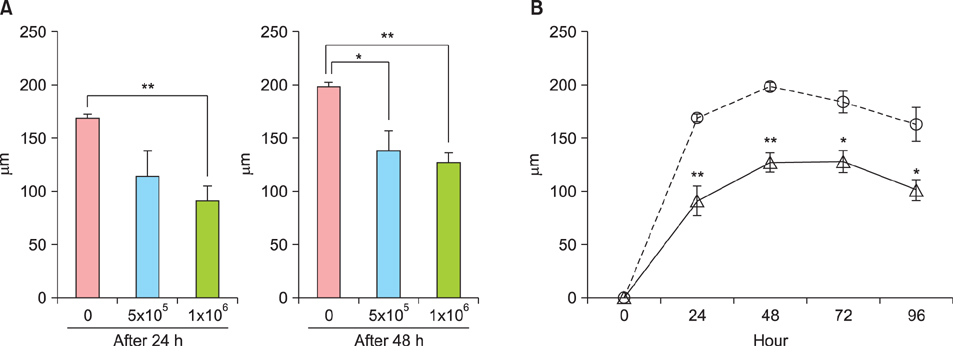
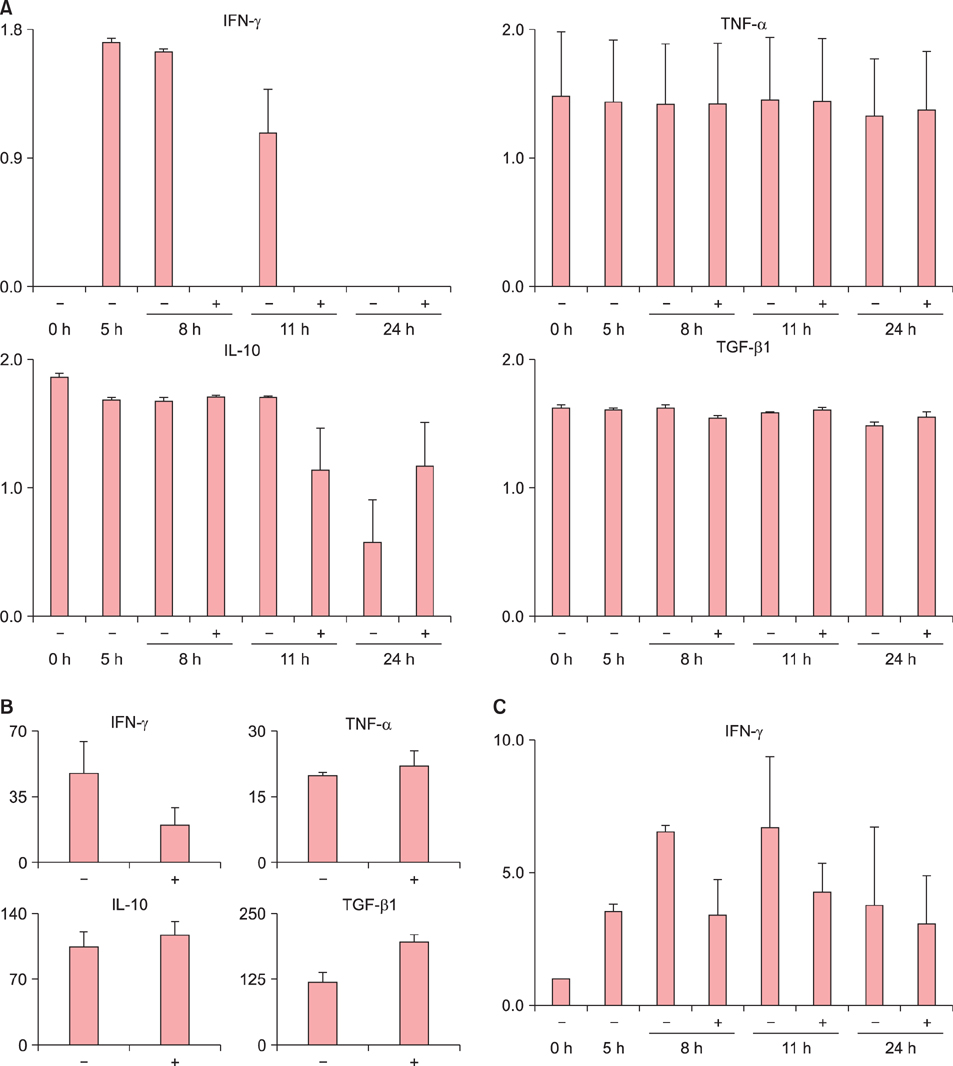
Ear thickness in a C57BL/6 mouse model of ACD using contact hypersensitivity (CHS) elicited by 2,4,6-trinitro-1-chlorobenzene was evaluated as a marker of inflammation level. Five and nine mice were injected with ASCs and phosphate-buffered saline (PBS), respectively. After ASC or PBS injection, real-time reverse transcription-polymerase chain reaction and enzyme-linked immunosorbent assay were performed.
RESULTS
Histology showed that CHS was self-limited and ear thickness was suppressed by ASCs in a dose-dependent manner. IFN-γ expression in the elicited skin site and regional lymph nodes was significantly lower in ASC-treated mice than in control mice. IL-10 expression did not differ between treated and control mice. The suppressive effects of ASCs on CHS response did not differ between IL-10 knock-out C57BL/6 mice and wild-type mice.
CONCLUSION
The present findings suggest that MSCs in adipose tissue may contribute to the self-limiting course of ACD through decreased expression of IFN-γ, but not through increased expression of IL-10.
Keyword
MeSH Terms
Figure
Reference
-
1. Saint-Mezard P, Rosieres A, Krasteva M, Berard F, Dubois B, Kaiserlian D, et al. Allergic contact dermatitis. Eur J Dermatol. 2004; 14:284–295.
Article2. Gaspari AA, Katz SI. Contact hypersensitivity. Curr Protoc Immunol. 2001; Chapter 4. Unit 4.2.
Article3. Tončić RJ, Lipozenčić J, Martinac I, Gregurić S. Immunology of allergic contact dermatitis. Acta Dermatovenerol Croat. 2011; 19:51–68.4. Sebastiani S, Albanesi C, De PO, Puddu P, Cavani A, Girolomoni G. The role of chemokines in allergic contact dermatitis. Arch Dermatol Res. 2002; 293:552–559.
Article5. Cavani A, Albanesi C, Traidl C, Sebastiani S, Girolomoni G. Effector and regulatory T cells in allergic contact dermatitis. Trends Immunol. 2001; 22:118–120.
Article6. Cavani A, Nasorri F, Prezzi C, Sebastiani S, Albanesi C, Girolomoni G. Human CD4+ T lymphocytes with remarkable regulatory functions on dendritic cells and nickel-specific Th1 immune responses. J Invest Dermatol. 2000; 114:295–302.
Article7. Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011; 109:923–940.8. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006; 8:315–317.
Article9. Konno M, Hamazaki TS, Fukuda S, Tokuhara M, Uchiyama H, Okazawa H, et al. Efficiently differentiating vascular endothelial cells from adipose tissue-derived mesenchymal stem cells in serum-free culture. Biochem Biophys Res Commun. 2010; 400:461–465.
Article10. Zhou Y, Yuan J, Zhou B, Lee AJ, Lee AJ, Ghawji M Jr, et al. The therapeutic efficacy of human adipose tissue-derived mesenchymal stem cells on experimental autoimmune hearing loss in mice. Immunology. 2011; 133:133–140.
Article11. Lin CS, Lin G, Lue TF. Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants. Stem Cells Dev. 2012; 21:2770–2778.
Article12. Fang B, Song Y, Liao L, Zhang Y, Zhao RC. Favorable response to human adipose tissue-derived mesenchymal stem cells in steroid-refractory acute graft-versus-host disease. Transplant Proc. 2007; 39:3358–3362.
Article13. Berg DJ, Leach MW, Kühn R, Rajewsky K, Müller W, Davidson NJ, et al. Interleukin 10 but not interleukin 4 is a natural suppressant of cutaneous inflammatory responses. J Exp Med. 1995; 182:99–108.
Article14. Bobr A, Igyarto BZ, Haley KM, Li MO, Flavell RA, Kaplan DH. Autocrine/paracrine TGF-β1 inhibits Langerhans cell migration. Proc Natl Acad Sci U S A. 2012; 109:10492–10497.
Article15. Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014; 14:478–494.
Article16. Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004; 22:460–466.
Article17. Mikami N, Matsushita H, Kato T, Kawasaki R, Sawazaki T, Kishimoto T, et al. Calcitonin gene-related peptide is an important regulator of cutaneous immunity: effect on dendritic cell and T cell functions. J Immunol. 2011; 186:6886–6893.
Article