Korean J Physiol Pharmacol.
2017 Sep;21(5):509-518. 10.4196/kjpp.2017.21.5.509.
Effect of glucose level on chemical hypoxia- and hydrogen peroxide-induced chemokine expression in human glioblastoma cell lines
- Affiliations
-
- 1Department of Physiology, Ewha Womans University School of Medicine, Seoul 07985, Korea. yc@ewha.ac.kr
- 2Tissue Injury Defense Research Center, Ewha Womans University School of Medicine, Seoul 07985, Korea.
- 3Department of Pathology, Ewha Womans University School of Medicine, Seoul 07985, Korea.
- KMID: 2388696
- DOI: http://doi.org/10.4196/kjpp.2017.21.5.509
Abstract
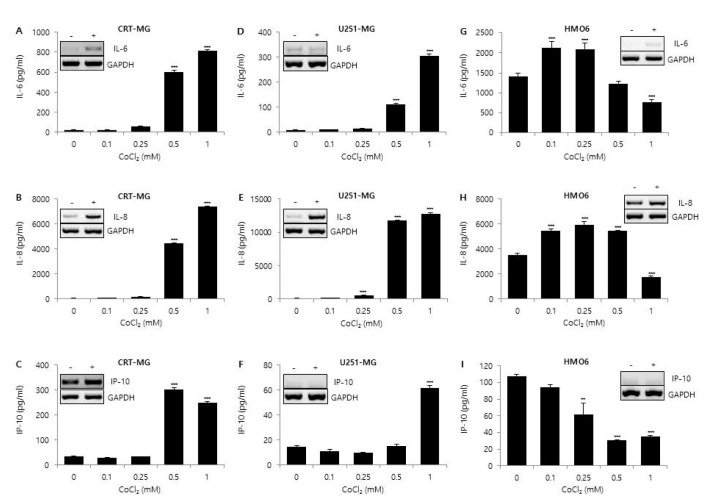
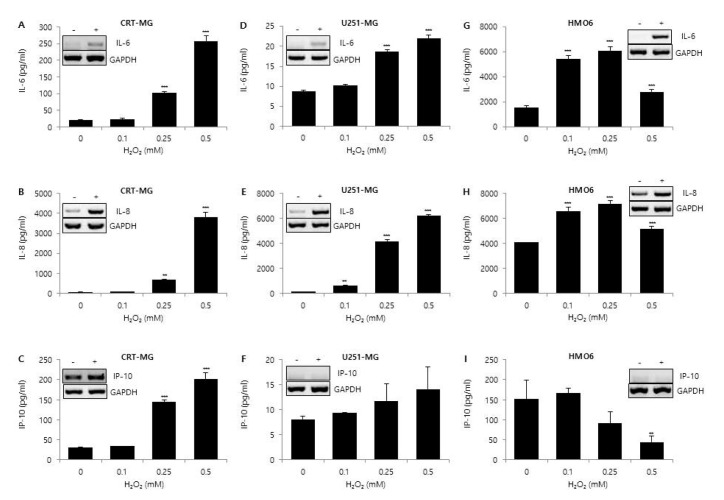
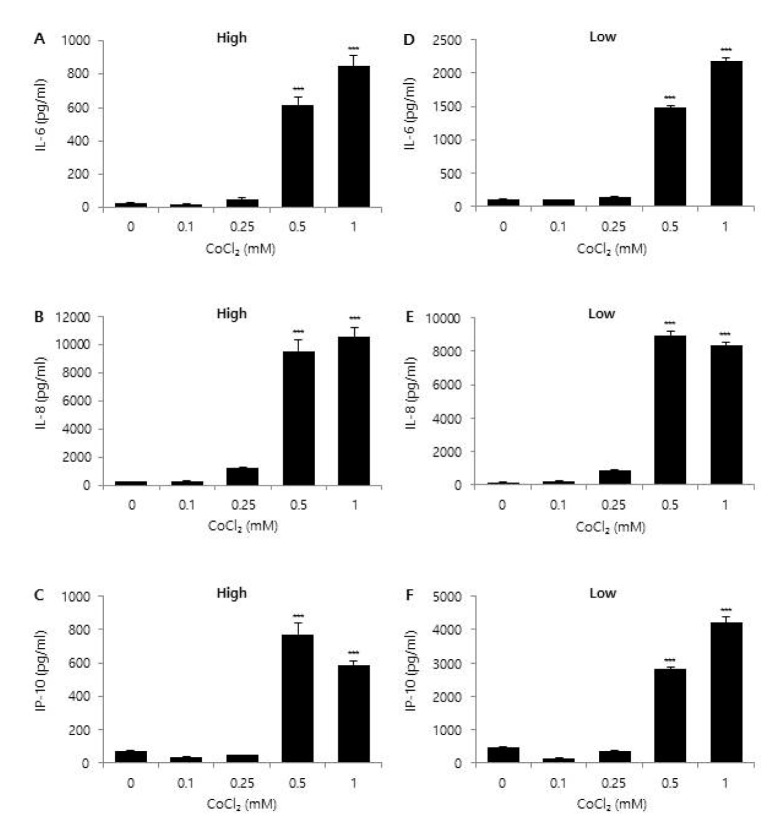
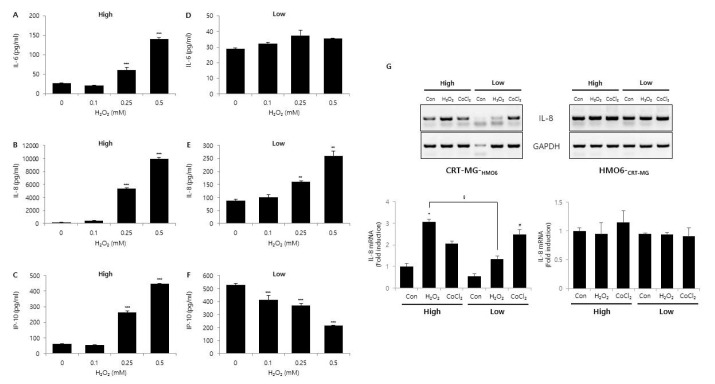
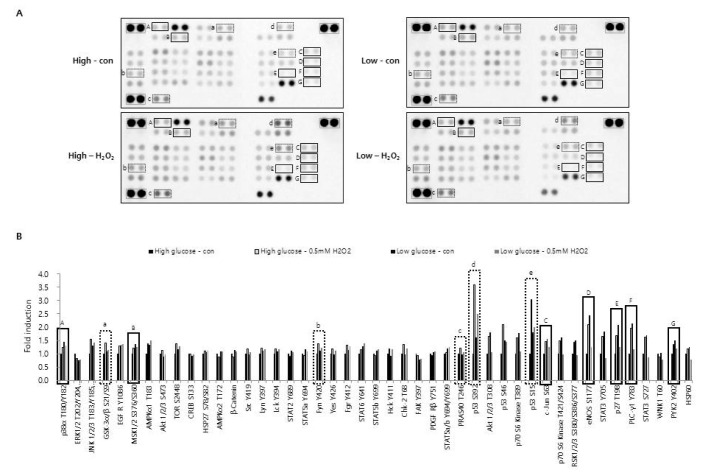
- Glioblastoma multiforme (GBM) is the most common primary intracranial tumor in adults and has poor prognosis. The GBM-specific tumor microenvironment (TME) plays a crucial role in tumor progression, immune escape, local invasion, and metastasis of GBM. Here, we demonstrate that hypoxia, reactive oxygen species (ROS), and differential concentration of glucose influence the expression of cytokines and chemokines, such as IL-6, IL-8, and IP-10, in human glial cell lines. Treatment with cobalt chloride (CoClâ‚‚) and hydrogen peroxide (Hâ‚‚Oâ‚‚) significantly increased the expression levels of IL-6, IL-8, and IP-10 in a dose-dependent manner in CRT-MG and U251-MG astroglioma cells, but not in microglia cells. However, we found strikingly different patterns of expression of cytokines and chemokines between Hâ‚‚Oâ‚‚-treated CRT-MG cells cultured in low- and high-glucose medium. These results suggest that astroglioma and microglia cells exhibit distinct patterns of cytokine and chemokine expression in response to CoClâ‚‚ and Hâ‚‚Oâ‚‚ treatment, and different concentrations of glucose influence this expression under either hypoxic or oxidant-enriched conditions.
Keyword
MeSH Terms
-
Adult
Anoxia
Astrocytoma
Cell Line*
Chemokines
Cobalt
Cytokines
Glioblastoma*
Glucose*
Humans*
Hydrogen Peroxide
Hydrogen*
Interleukin-6
Interleukin-8
Microglia
Neoplasm Metastasis
Neuroglia
Prognosis
Reactive Oxygen Species
Tumor Microenvironment
United Nations
Chemokines
Cobalt
Cytokines
Glucose
Hydrogen
Hydrogen Peroxide
Interleukin-6
Interleukin-8
Reactive Oxygen Species
Figure
Reference
-
1. Friedmann-Morvinski D, Narasimamurthy R, Xia Y, Myskiw C, Soda Y, Verma IM. Targeting NF-κB in glioblastoma: a therapeutic approach. Sci Adv. 2016; 2:e1501292. PMID: 26824076.
Article2. Kupp R, Shtayer L, Tien AC, Szeto E, Sanai N, Rowitch DH, Mehta S. Lineage-restricted OLIG2-RTK signaling governs the molecular subtype of glioma stem-like cells. Cell Rep. 2016; 16:2838–2845. PMID: 27626655.
Article3. McLendon RE, Halperin EC. Is the long-term survival of patients with intracranial glioblastoma multiforme overstated? Cancer. 2003; 98:1745–1748. PMID: 14534892.
Article4. Puli S, Lai JC, Bhushan A. Inhibition of matrix degrading enzymes and invasion in human glioblastoma (U87MG) cells by isoflavones. J Neurooncol. 2006; 79:135–142. PMID: 16598420.
Article5. Kesari S. Understanding glioblastoma tumor biology: the potential to improve current diagnosis and treatments. Semin Oncol. 2011; 38(Suppl 4):S2–S10. PMID: 22078644.
Article6. Mammoto T, Jiang A, Jiang E, Panigrahy D, Kieran MW, Mammoto A. Role of collagen matrix in tumor angiogenesis and glioblastoma multiforme progression. Am J Pathol. 2013; 183:1293–1305. PMID: 23928381.
Article7. Codrici E, Enciu AM, Popescu ID, Mihai S, Tanase C. Glioma stem cells and their microenvironments: providers of challenging therapeutic targets. Stem Cells Int. 2016; 2016:5728438. PMID: 26977157.
Article8. Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst. 2007; 99:1583–1593. PMID: 17971532.
Article9. Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev. 2008; 18:27–34. PMID: 18282701.
Article10. Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006; 26:5336–5347. PMID: 16809770.
Article11. Chen JL, Lucas JE, Schroeder T, Mori S, Wu J, Nevins J, Dewhirst M, West M, Chi JT. The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genet. 2008; 4:e1000293. PMID: 19057672.
Article12. Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res. 2010; 16:5928–5935. PMID: 20962028.
Article13. Fiaschi T, Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol. 2012; 2012:762825. PMID: 22666258.
Article14. Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J, Nishioka Y, Sone S, Kuwano M. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int J Cancer. 2000; 85:182–188. PMID: 10629075.15. Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001; 193:727–740. PMID: 11257139.
Article16. Kim J, Takeuchi H, Lam ST, Turner RR, Wang HJ, Kuo C, Foshag L, Bilchik AJ, Hoon DS. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005; 23:2744–2753. PMID: 15837989.17. Schadendorf D, Möller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993; 151:2667–2675. PMID: 8360485.18. Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005; 7:122–133. PMID: 15831231.19. Maru SV, Holloway KA, Flynn G, Lancashire CL, Loughlin AJ, Male DK, Romero IA. Chemokine production and chemokine receptor expression by human glioma cells: role of CXCL10 in tumour cell proliferation. J Neuroimmunol. 2008; 199:35–45. PMID: 18538864.
Article20. Michaud-Levesque J, Bousquet-Gagnon N, Béliveau R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp Cell Res. 2012; 318:925–935. PMID: 22394507.
Article21. Shanmugam N, Reddy MA, Guha M, Natarajan R. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes. 2003; 52:1256–1264. PMID: 12716761.
Article22. Weng L, Zhang H, Li X, Zhan H, Chen F, Han L, Xu Y, Cao X. Ampelopsin attenuates lipopolysaccharide-induced inflammatory response through the inhibition of the NF-kB and JAK2/STAT3 signaling pathways in microglia. Int Immunopharmacol. 2017; 44:1–8. PMID: 27998743.23. Hsieh HL, Yang CM. Astrocyte as the modulator in brain inflammation and neurodegenerative disorders. Ann Neurodegener Disord. 2016; 1:1008.24. Bélanger M, Magistretti PJ. The role of astroglia in neuroprotection. Dialogues Clin Neurosci. 2009; 11:281–295. PMID: 19877496.
Article25. Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010; 7:22–30. PMID: 20129494.
Article26. Ahn SH, Park H, Ahn YH, Kim S, Cho MS, Kang JL, Choi YH. Necrotic cells influence migration and invasion of glioblastoma via NF-κB/AP-1-mediated IL-8 regulation. Sci Rep. 2016; 6:24552. PMID: 27076368.
Article27. Lindemann C, Marschall V, Weigert A, Klingebiel T, Fulda S. Smac mimetic-induced upregulation of CCL2/MCP-1 triggers migration and invasion of glioblastoma cells and influences the tumor microenvironment in a paracrine manner. Neoplasia. 2015; 17:481–489. PMID: 26152356.
Article28. Sharma I, Siraj F, Sharma KC, Singh A. Immunohistochemical expression of chemokine receptor CXCR3 and its ligand CXCL10 in low-grade astrocytomas and glioblastoma multiforme: a tissue microarray-based comparison. J Cancer Res Ther. 2016; 12:793–797. PMID: 27461653.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoxia induces Wee1 expression and attenuates hydrogen peroxide-induced endothelial damage in MS1 cells
- Hydrogen peroxide mediates doxorubicin-induced transglutaminase 2 expression in PC-14 human lung cancer cell line
- Enhanced Expression of Glucose 2-Oxidase in Phlebia tremellosa by Addition of Phthalates
- A case of hydrogen peroxide-induced proctocolitis in a child
- The Effect of Induced Heat Shock Protein 33 in Human Corneal Epithelial Cell